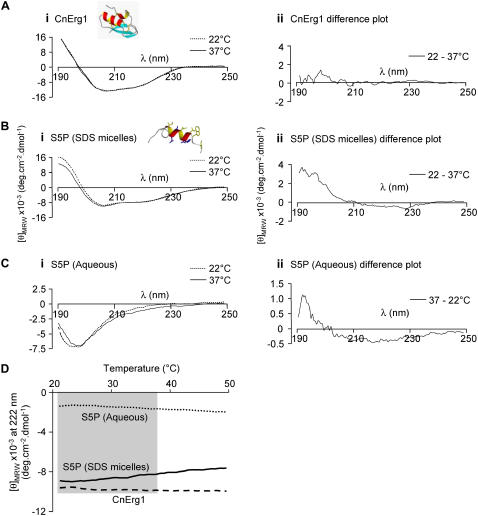
FIGURE 12.
CD spectra for (A) CnErg1, (B) hERG S5P helix peptide in SDS micelles, and (C) hERG S5P peptide in aqueous solution, at 22°C and 37°C. Insets to panels A (i) and B (i) show the structures of the respective peptides, determined by two-dimensional NMR spectroscopy. Panel (ii) shows the difference spectrum for each peptide and condition. (D) Thermal melt curves for CnErg1, hERG S5P helix peptide in SDS micelles, and hERG S5P helix peptide in water. The shaded area indicates the temperature range 22°C to 37°C corresponding to the temperatures at which the kinetics of CnErg1 binding were assayed in this study. Over this range the α-helix content of the CnErg1 peptide (measured from the negative molar ellipticity at 222 nm) is essentially unchanged (as it is over the entire range tested). Between 22°C and 37°C the α-helix content of the hERG S5P helix peptide steadily increases in the aqueous solution ([θ]MRW increases from −1420 to −1740 deg cm−2 dmol−1) but decreases in SDS micelles ([θ]MRW decreases from −9050 to −8340 deg cm−2 dmol−1).