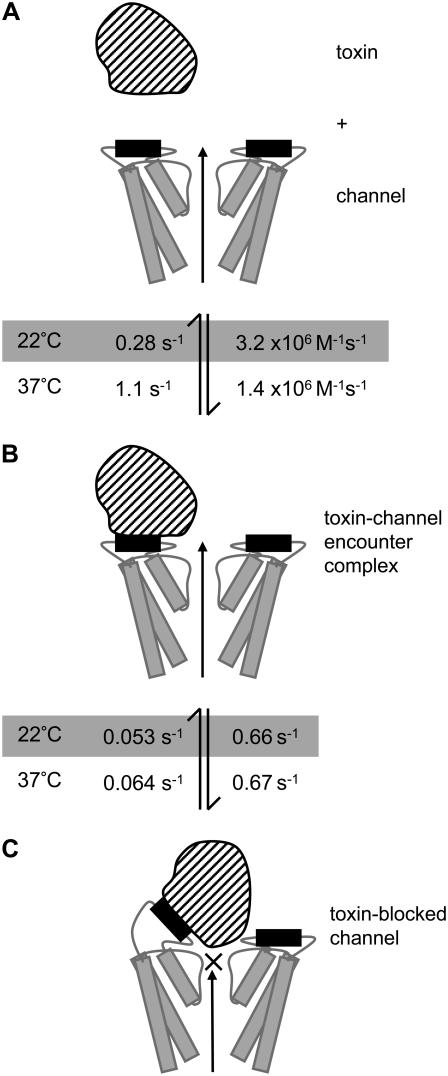
FIGURE 13.
Cartoon depicting proposed model of CnErg1 binding to hERG. Panel A depicts the free toxin (striped) and channel (two subunits shown) drawn approximately to scale based on the NMR structure for CnErg1 (22) and crystal structure of KcsA (46). The hERG S5P amphipathic α-helix, that forms part of the CnErg1 binding site, is shown in black. Panel B depicts the toxin channel encounter complex (TC* in Scheme 2). Panel C depicts the toxin-blocked channel (TC in Scheme 2). The rate constants at 22°C (shaded) and at 37°C are those shown in Table 1. The two important features of the scheme are that 1), the initial encounter of toxin with the channel occurs at a site that does not overlap the central axis and so permits ion conduction, depicted by the long arrow; and 2), the toxin-channel encounter complex undergoes a conformational change that results in occlusion of the pore. The nature of that conformational change, however, is speculative. In this model, the temperature-dependence of CnErg1 binding to hERG is explained by the hERG S5P amphipathic α-helix (putative CnErg1 binding site) being thermally labile and so increasing temperature primarily affects the values for k+1 and k−1.