Figure 5.
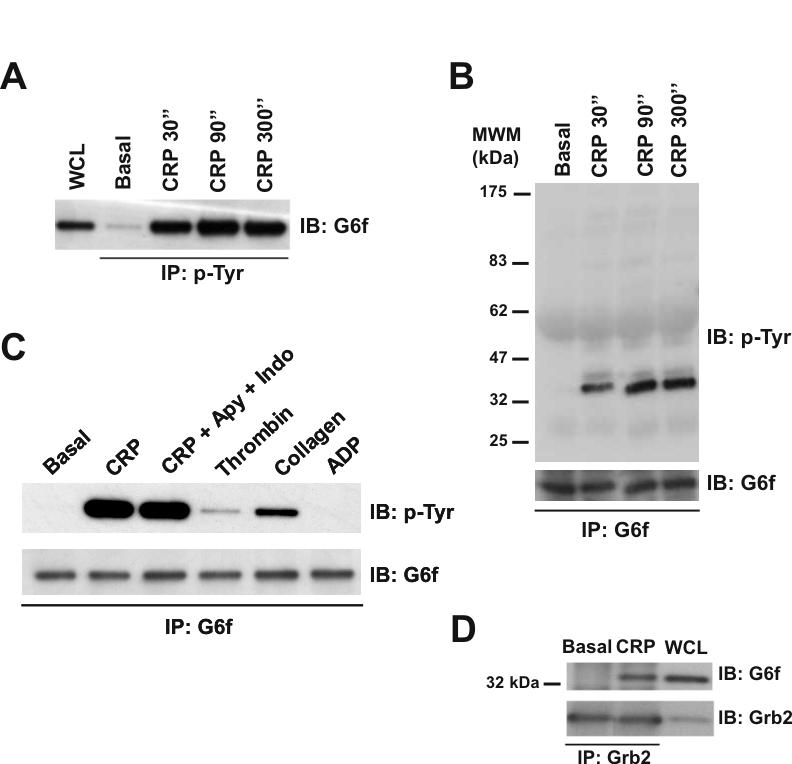
G6f is phosphorylated in Tyr-281 and is able to bind Grb2 in GPVI-stimulated platelets. (A) Tyrosine-phosphorylated G6f is recruited into the GPVI signaling cascade upon platelet stimulation with CRP (10 μg/mL), with maximum levels of phosphorylated G6f being observed after 90 sec stimulation. Whole cell lysates and phosphotyrosine immunoprecipitated proteins were separated by 4-12% NuPAGE Bis-Tris gels and immunoblotted using an anti-G6f antibody. (B) Time-course experiments for CRP-platelet stimulation confirm that G6f is tyrosine-phosphorylated upon platelet-activation with CRP (10 μg/mL). G6f immunoprecipitated proteins were separated by 4-12% NuPAGE Bis-Tris gels and immunoblotted using anti-4G10 and then anti-G6f antibodies. (C) G6f undergoes tyrosine-phosphorylation downstream of GPVI but not by the G protein-coupled receptor agonists thrombin and ADP. G6f immunoprecipitated proteins were separated by 4-12% NuPAGE Bis-Tris gels and immunoblotted using anti-4G10 and anti-G6f antibodies. All platelets had EGTA added to prevent aggregation; only where specified have apyrase (2 U/mL) and indomethacin (10 μM) been added. Platelets were stimulated for 90 sec with CRP (10 μg/mL), collagen (30 μg/mL), thrombin (1 U/mL), and ADP (100 μM). (D) G6f co-immunoprecipitates with Grb2 in CRP-stimulated platelets. Whole cell lysate and Grb2 immunoprecipitated proteins were separated by 4-12% NuPAGE Bis-Tris gels and immunoblotted using anti G6f and then anti-Grb2 antibodies. Platelets were stimulated with CRP (10 μg/mL, 90 sec). IP, immunoprecipitation; IB, immuno blot; WCL, whole cell lysate. Images represent at least three independent experiments.
