Abstract
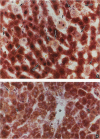
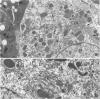
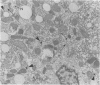
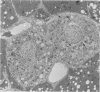
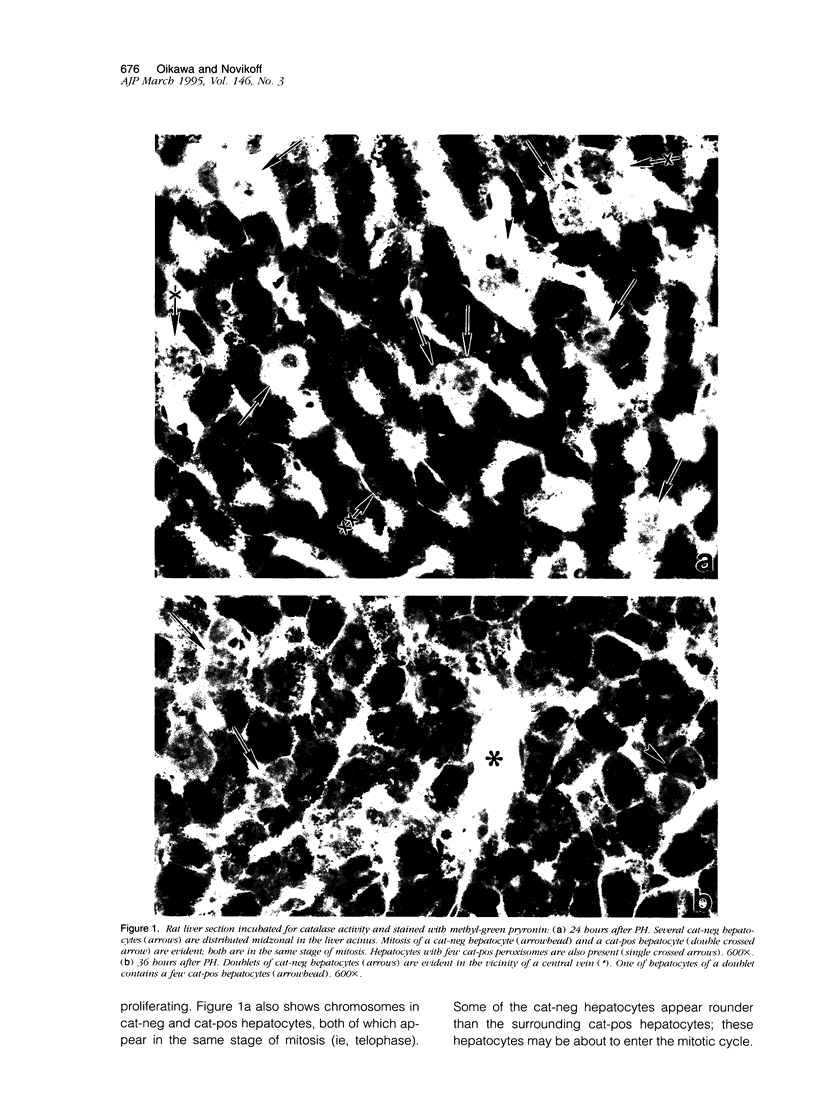
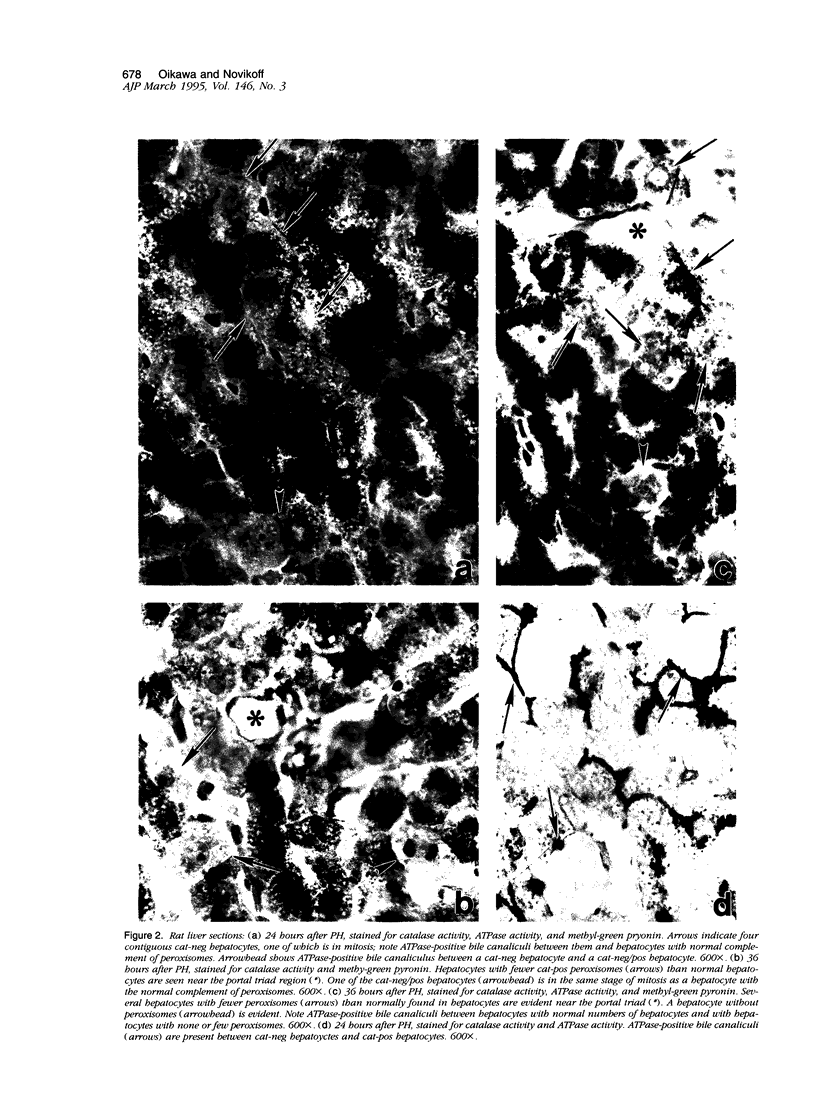
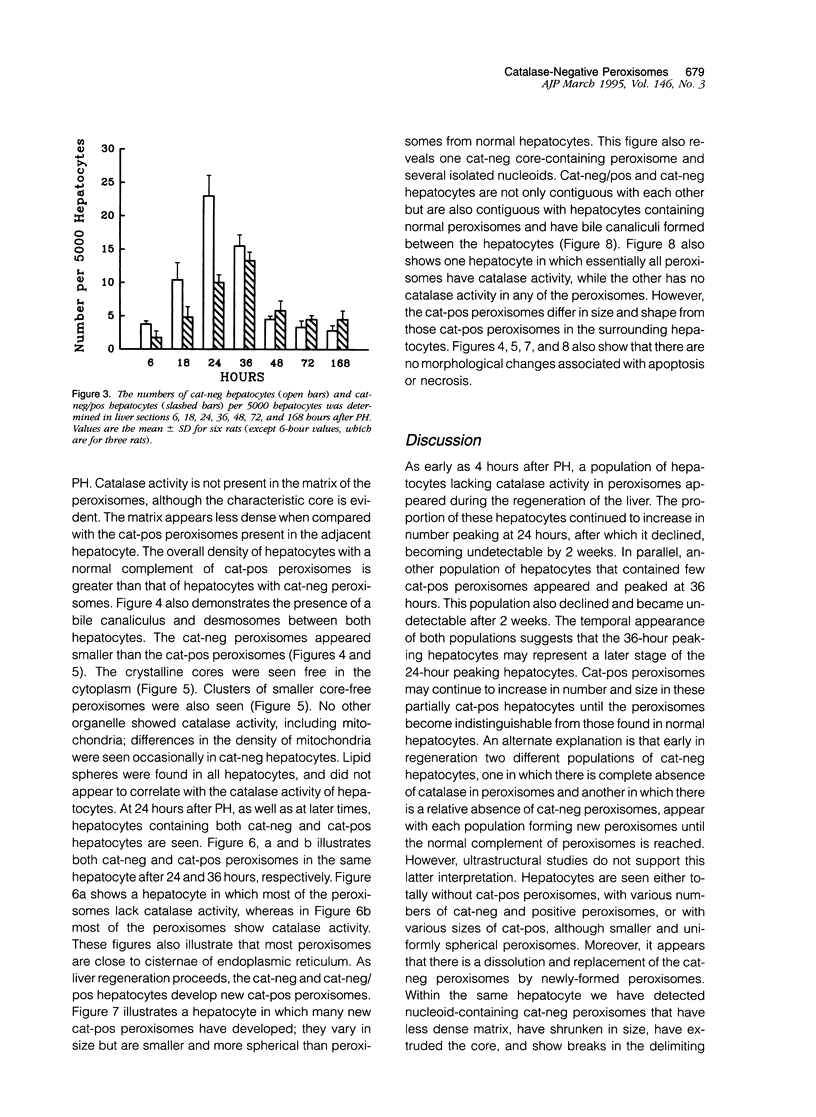
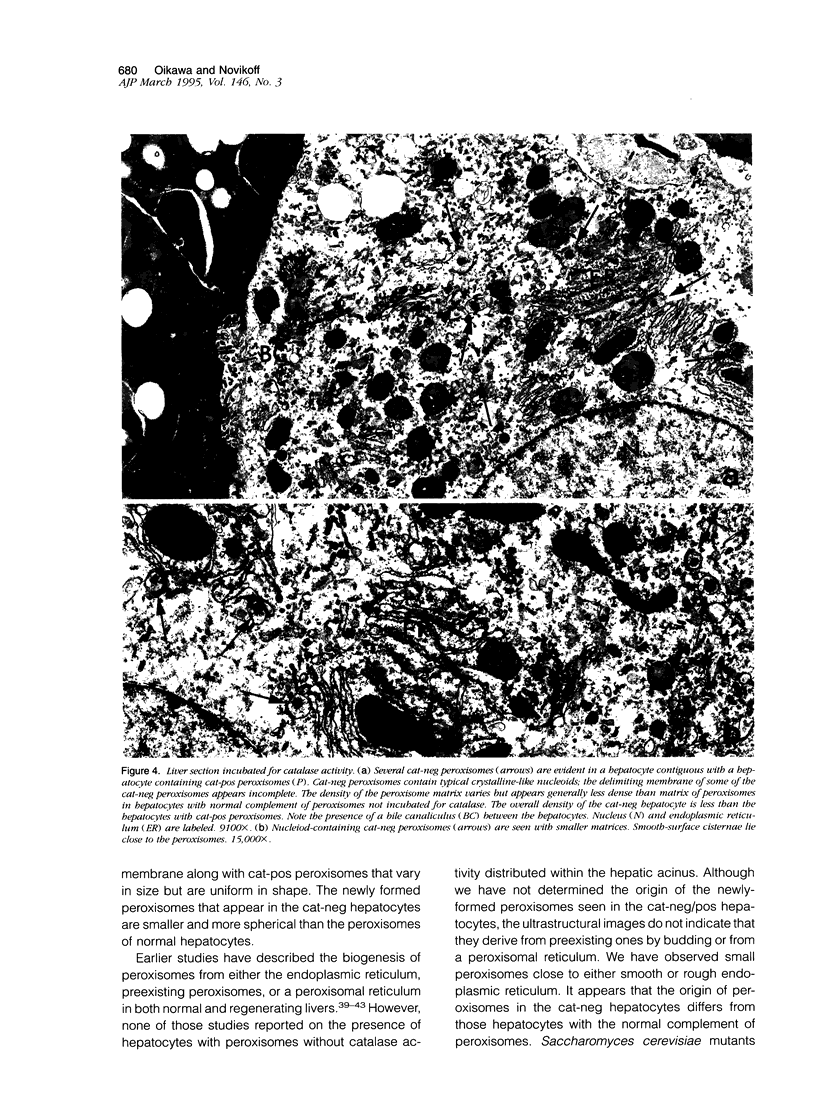
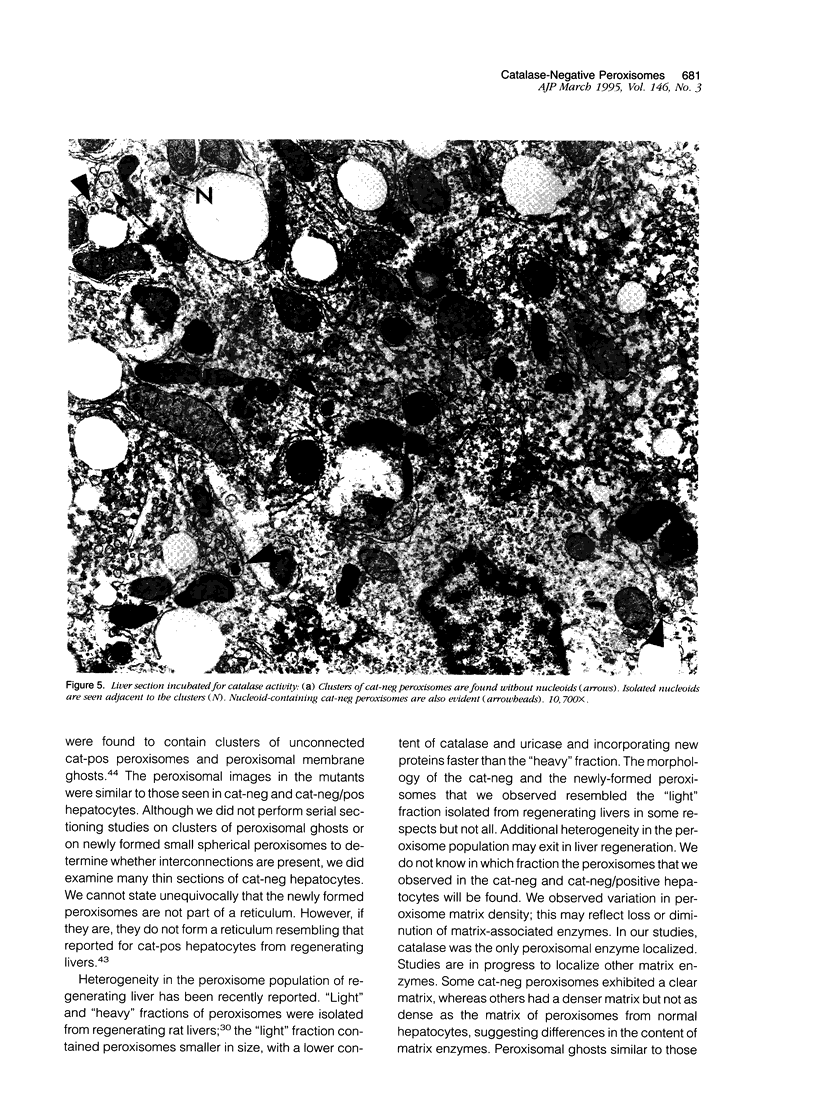
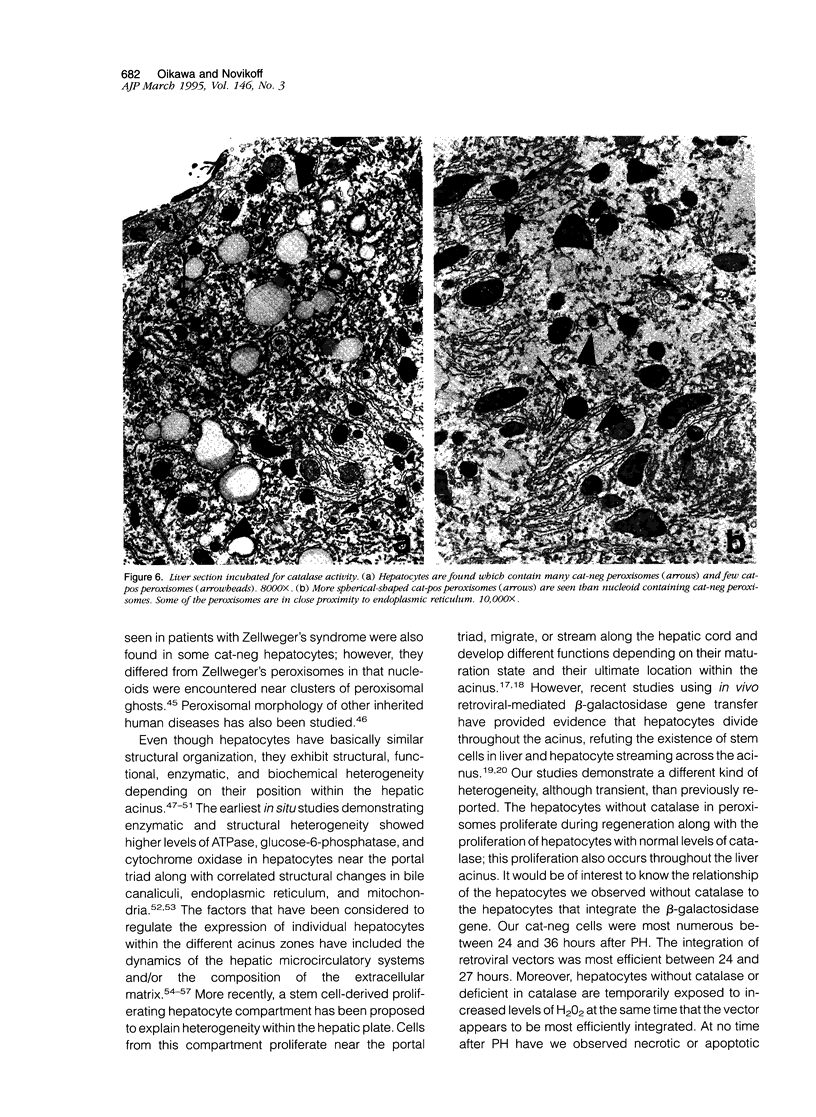
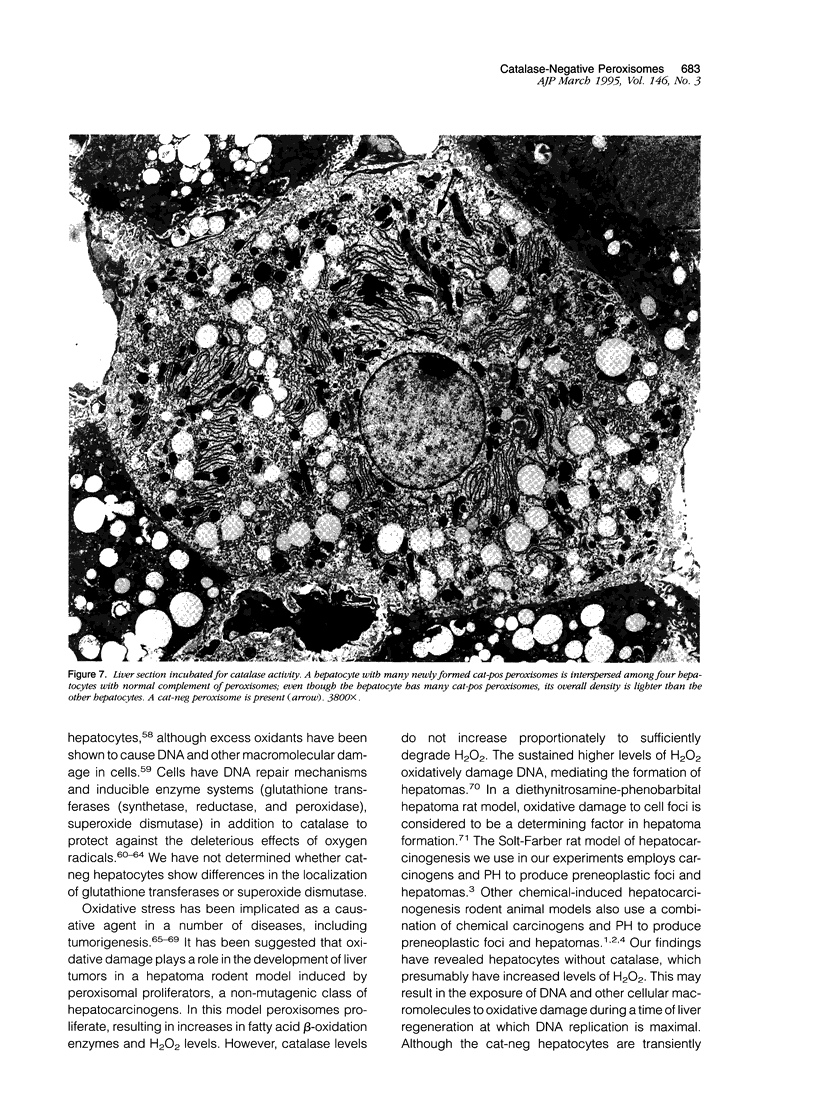
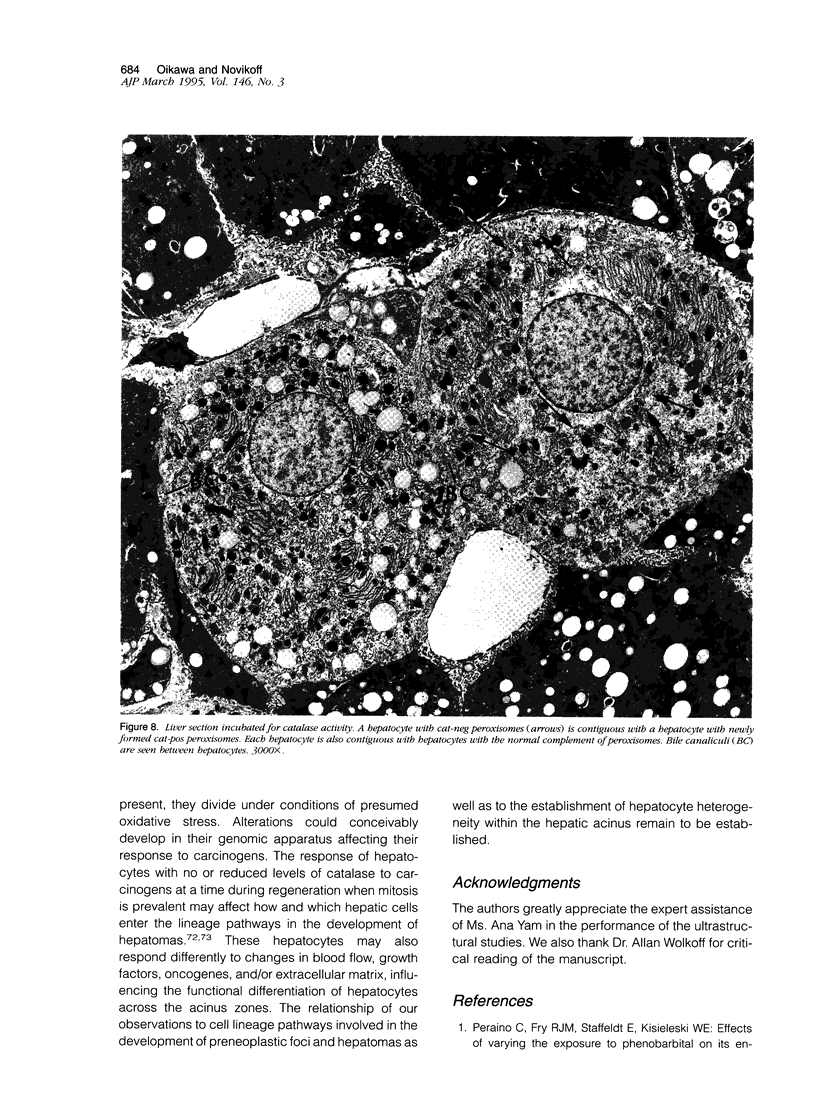
Using light microscopy enzyme cytochemistry to localize catalase activity in peroxisomes, a population of peroxisome-negative hepatocytes was detected in livers of rats during liver regeneration induced by two-thirds partial hepatectomy. However, examination by electron microscopy revealed that this population of hepatocytes contained peroxisomes with a delimiting membrane and a nucleoid, but no cytochemically demonstrable catalase activity within their matrix. Regenerating livers 6, 18, 24, 36, 48 and 72 hours, and 1 week after partial hepatectomy showed hepatocytes without catalase activity. However, their numbers varied, with the most numerous appearing at 24 hours after partial hepatectomy. Mitosis of catalase-negative hepatocytes were seen along with mitosis of hepatocytes containing the normal complement of catalase-positive peroxisomes. The catalase-negative hepatocytes did not show evidence of apoptosis or necrotic cell death. Lysosomal acid phosphatase activity and bile canalicular ATPase activity were present in hepatocytes with catalase-negative peroxisomes. Another population of hepatocytes with a small number of catalase-positive peroxisomes appeared and were more numerous at 36 hours after partial hepatectomy; ultrastructurally, these hepatocytes contained both catalase-negative peroxisomes, which appeared to undergo dissolution, and catalase-positive peroxisomes, which were smaller in size. After complete restoration of the liver, all hepatocytes displayed essentially uniform numbers of catalase-positive peroxisomes. These studies indicated that during liver regeneration there is a transient loss of catalase in peroxisomes of some hepatocytes. These cells proliferate and with time acquire new catalase-positive peroxisomes. The observations are discussed in relation to peroxisome biogenesis, hepatocellular carcinogenesis, and oxidative stress during liver regeneration.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber N., Zajicek G., Ariel I. The streaming liver. II. Hepatocyte life history. Liver. 1988 Apr;8(2):80–87. doi: 10.1111/j.1600-0676.1988.tb00972.x. [DOI] [PubMed] [Google Scholar]
- Baumgart E., Völkl A., Hashimoto T., Fahimi H. D. Biogenesis of peroxisomes: immunocytochemical investigation of peroxisomal membrane proteins in proliferating rat liver peroxisomes and in catalase-negative membrane loops. J Cell Biol. 1989 Jun;108(6):2221–2231. doi: 10.1083/jcb.108.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bralet M. P., Branchereau S., Brechot C., Ferry N. Cell lineage study in the liver using retroviral mediated gene transfer. Evidence against the streaming of hepatocytes in normal liver. Am J Pathol. 1994 May;144(5):896–905. [PMC free article] [PubMed] [Google Scholar]
- Coleman W. B., Wennerberg A. E., Smith G. J., Grisham J. W. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993 May;142(5):1373–1382. [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Farr S. B., Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991 Dec;55(4):561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N. Hepatocyte differentiation and liver progenitor cells. Curr Opin Cell Biol. 1990 Dec;2(6):1036–1042. doi: 10.1016/0955-0674(90)90153-6. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990 Jun;4(9):2587–2597. [PubMed] [Google Scholar]
- Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol Ther. 1992;53(1):127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- Goldenberg H., Hüttinger M., Böck P., Kramar R. Influence of subtotal hepatectomy on peroxisomes and peroxisomal enzymes of rat liver and isolated liver cell fractions. Histochemistry. 1975 Jul 16;44(1):47–56. doi: 10.1007/BF00490420. [DOI] [PubMed] [Google Scholar]
- Goldsworthy T. L., Hanigan M. H., Pitot H. C. Models of hepatocarcinogenesis in the rat--contrasts and comparisons. Crit Rev Toxicol. 1986;17(1):61–89. doi: 10.3109/10408448609037071. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Grisham J. W. Migration of hepatocytes along hepatic plates and stem cell-fed hepatocyte lineages. Am J Pathol. 1994 May;144(5):849–854. [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989 Jul;69(3):708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Ketterer B., Meyer D. J. Glutathione transferases: a possible role in the detoxication and repair of DNA and lipid hydroperoxides. Mutat Res. 1989 Sep;214(1):33–40. doi: 10.1016/0027-5107(89)90195-4. [DOI] [PubMed] [Google Scholar]
- Kren B. T., Kumar N. M., Wang S. Q., Gilula N. B., Steer C. J. Differential regulation of multiple gap junction transcripts and proteins during rat liver regeneration. J Cell Biol. 1993 Nov;123(3):707–718. doi: 10.1083/jcb.123.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Le Hir M., Dubach U. C. The activities of peroxisomal oxidases in periportal and perivenous zones of the rat liver acinus. Histochemistry. 1980;69(1):95–99. doi: 10.1007/BF00508370. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991 Sep;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Lüers G., Hashimoto T., Fahimi H. D., Völkl A. Biogenesis of peroxisomes: isolation and characterization of two distinct peroxisomal populations from normal and regenerating rat liver. J Cell Biol. 1993 Jun;121(6):1271–1280. doi: 10.1083/jcb.121.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Delgado F. M., Amenta P. S. The extracellular matrix in hepatic regeneration. Localization of collagen types I, III, IV, laminin, and fibronectin. Lab Invest. 1991 Feb;64(2):157–166. [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984 Jul;51(1):57–74. [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- NOVIKOFF A. B. Cell heterogeneity within the hepatic lobule of the rat: staining reactions. J Histochem Cytochem. 1959 Jul;7(4):240–244. doi: 10.1177/7.4.240. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., ESSNER E. The liver cell. Some new approaches to its study. Am J Med. 1960 Jul;29:102–131. doi: 10.1016/0002-9343(60)90011-5. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M., Davis C., Quintana N. Studies on microperoxisomes. II. A cytochemical method for light and electron microscopy. J Histochem Cytochem. 1972 Dec;20(12):1006–1023. doi: 10.1177/20.12.1006. [DOI] [PubMed] [Google Scholar]
- Novikoff A. B. The endoplasmic reticulum: a cytochemist's view (a review). Proc Natl Acad Sci U S A. 1976 Aug;73(8):2781–2787. doi: 10.1073/pnas.73.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S. Oxidative DNA damage caused by persistent peroxisome proliferation: its role in hepatocarcinogenesis. Mutat Res. 1989 Sep;214(1):63–68. doi: 10.1016/0027-5107(89)90198-x. [DOI] [PubMed] [Google Scholar]
- Reddy J., Svoboda D. Microbodies in experimentally altered cells. 8. Continuities between microbodies and their possible biologic significance. Lab Invest. 1971 Jan;24(1):74–81. [PubMed] [Google Scholar]
- Reid L. M. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990 Feb;2(1):121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- Rigatuso J. L., Legg P. G., Wood R. L. Microbody formation in regenerating rat liver. J Histochem Cytochem. 1970 Dec;18(12):893–900. doi: 10.1177/18.12.893. [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Spray D. C., Reid L. M. Transcriptional and posttranscriptional control of connexin mRNAs in periportal and pericentral rat hepatocytes. Eur J Cell Biol. 1992 Oct;59(1):21–26. [PubMed] [Google Scholar]
- Santos M. J., Imanaka T., Shio H., Lazarow P. B. Peroxisomal integral membrane proteins in control and Zellweger fibroblasts. J Biol Chem. 1988 Jul 25;263(21):10502–10509. [PubMed] [Google Scholar]
- Scherer E., Emmelot P. Kinetics of induction and growth of precancerous liver-cell foci, and liver tumour formation by diethylnitrosamine in the rat. Eur J Cancer. 1975 Oct;11(10):689–696. doi: 10.1016/0014-2964(75)90042-0. [DOI] [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Sell S. The role of determined stem-cells in the cellular lineage of hepatocellular carcinoma. Int J Dev Biol. 1993 Mar;37(1):189–201. [PubMed] [Google Scholar]
- Sigal S. H., Brill S., Fiorino A. S., Reid L. M. The liver as a stem cell and lineage system. Am J Physiol. 1992 Aug;263(2 Pt 1):G139–G148. doi: 10.1152/ajpgi.1992.263.2.G139. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Mathis G. A., Sano N., Elmore L. W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58(1):44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Solt D. B., Medline A., Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977 Sep;88(3):595–618. [PMC free article] [PubMed] [Google Scholar]
- Stark A. A., Russell J. J., Langenbach R., Pagano D. A., Zeiger E., Huberman E. Localization of oxidative damage by a glutathione-gamma-glutamyl transpeptidase system in preneoplastic lesions in sections of livers from carcinogen-treated rats. Carcinogenesis. 1994 Feb;15(2):343–348. doi: 10.1093/carcin/15.2.343. [DOI] [PubMed] [Google Scholar]
- Sternlieb I., Quintana N. The peroxisomes of human hepatocytes. Lab Invest. 1977 Feb;36(2):140–149. [PubMed] [Google Scholar]
- Teebor G. W., Boorstein R. J., Cadet J. The repairability of oxidative free radical mediated damage to DNA: a review. Int J Radiat Biol. 1988 Aug;54(2):131–150. doi: 10.1080/09553008814551591. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson S. S. Hepatic stem cells. Am J Pathol. 1993 May;142(5):1331–1333. [PMC free article] [PubMed] [Google Scholar]
- Traber P. G., Chianale J., Gumucio J. J. Physiologic significance and regulation of hepatocellular heterogeneity. Gastroenterology. 1988 Oct;95(4):1130–1143. doi: 10.1016/0016-5085(88)90194-1. [DOI] [PubMed] [Google Scholar]
- WACHSTEIN M., MEISEL E. Histochemistry of hepatic phosphatases of a physiologic pH; with special reference to the demonstration of bile canaliculi. Am J Clin Pathol. 1957 Jan;27(1):13–23. doi: 10.1093/ajcp/27.1.13. [DOI] [PubMed] [Google Scholar]
- WILSON J. W., LEDUC E. H. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958 Oct;76(2):441–449. doi: 10.1002/path.1700760213. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Fahimi H. D. Biogenesis of peroxisomes in regenerating rat liver. I. Sequential changes of catalase and urate oxidase detected by ultrastructural cytochemistry. Eur J Cell Biol. 1987 Jun;43(3):293–300. [PubMed] [Google Scholar]
- Yamamoto K., Fahimi H. D. Three-dimensional reconstruction of a peroxisomal reticulum in regenerating rat liver: evidence of interconnections between heterogeneous segments. J Cell Biol. 1987 Aug;105(2):713–722. doi: 10.1083/jcb.105.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef P. P., York J. R. Septic arthritis: a second decade of experience. Aust N Z J Med. 1994 Jun;24(3):307–311. doi: 10.1111/j.1445-5994.1994.tb02178.x. [DOI] [PubMed] [Google Scholar]
- Zhang J. W., Han Y., Lazarow P. B. Novel peroxisome clustering mutants and peroxisome biogenesis mutants of Saccharomyces cerevisiae. J Cell Biol. 1993 Dec;123(5):1133–1147. doi: 10.1083/jcb.123.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch H., Schutgens R. B., Wanders R. J., Tager J. M. Biochemistry of peroxisomes. Annu Rev Biochem. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]