Abstract
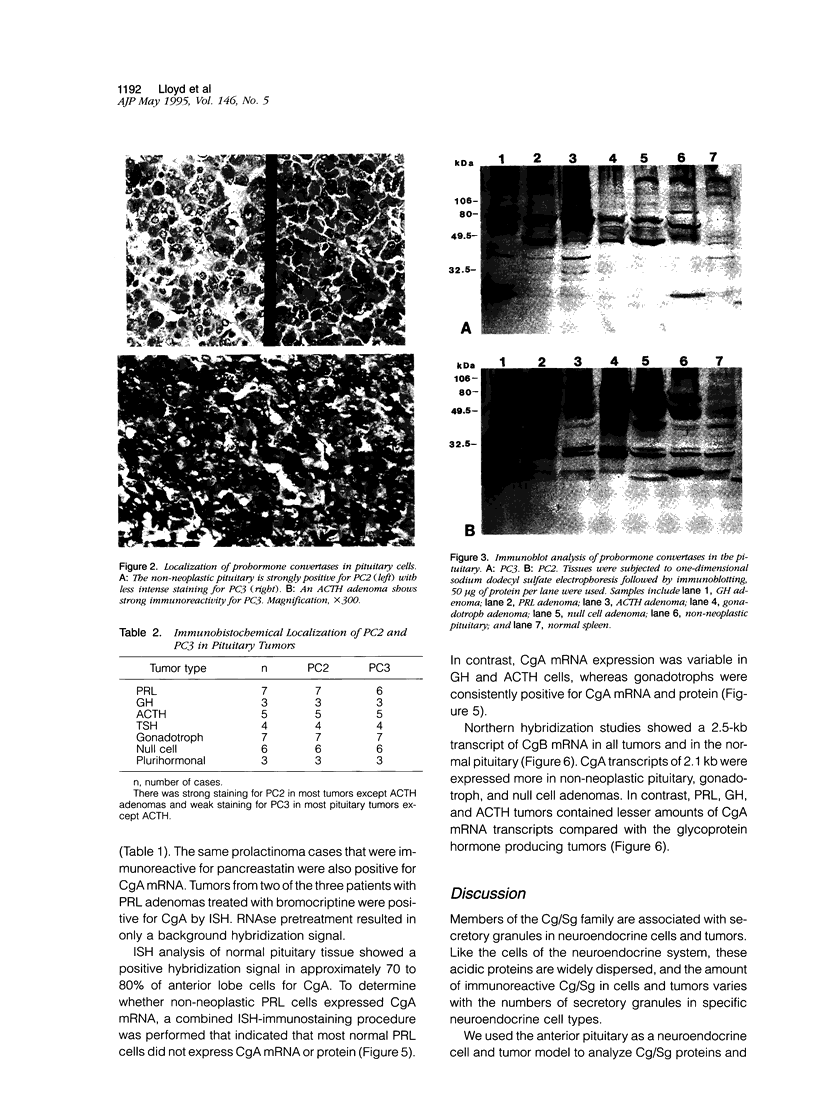
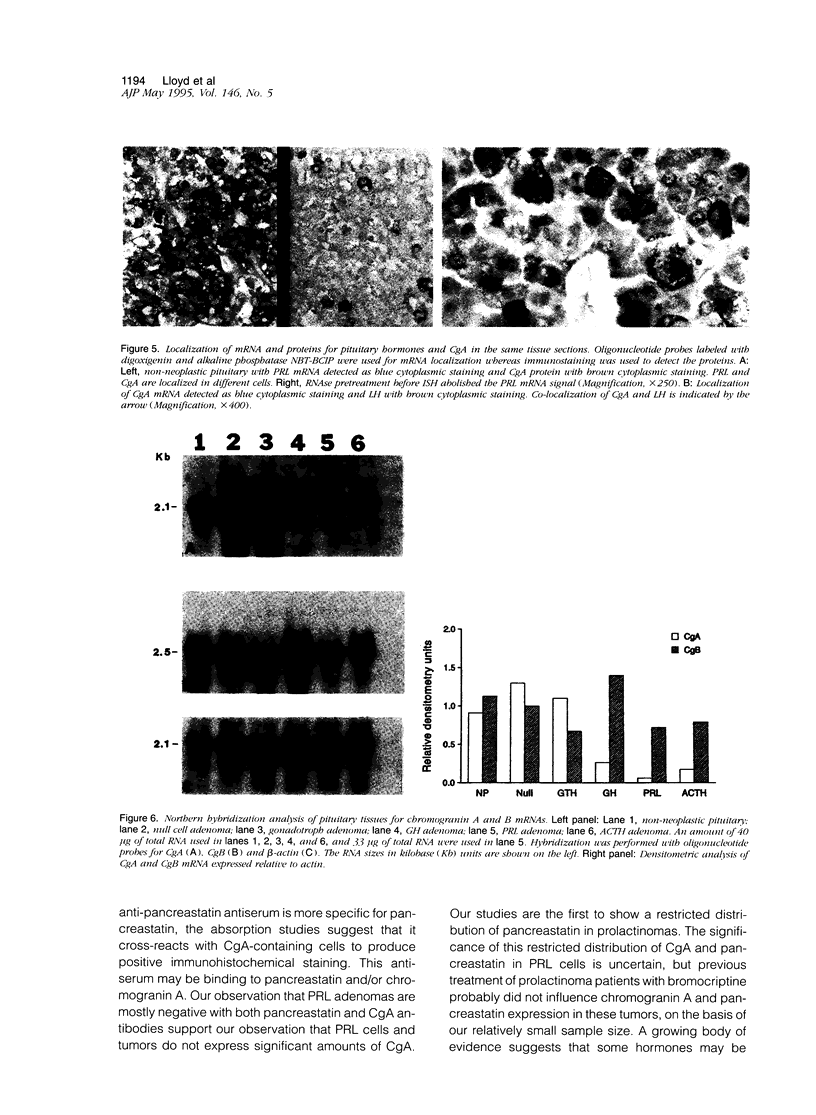
Several members of the chromogranin/secretogranin (Cg/Sg) family are post-translationally processed in neuroendocrine cells and tumors to smaller peptides, some of which are biologically active. For example, CgA is processed to pancreastatin, parastatin, and other peptides. We analyzed the distribution of pancreastatin and CgA proteins in normal and neoplastic pituitaries as well as the prohormone convertases PC2 and PC3/1 (PC3), the putative processing enzymes for the Cg/Sg family, in 35 pituitary adenomas and 4 non-neoplastic pituitaries by immunohistochemistry and immunoblotting with highly specific antisera. CgA and CgB mRNAs were also examined. Pancreastatin was present in all subtypes of pituitary tumors, although prolactin-secreting adenomas expressed this peptide less frequently than did other tumor types. CgA protein and CgA mRNA expression were also restricted in prolactin adenomas and in normal prolactin cells, as shown by combined in situ hybridization and immunostaining. The prohormone convertases PC2 and PC3 were present in pituitary tumors and in non-neoplastic pituitaries. Immunoblot analysis and immunostaining showed a principal approximately 69-kd PC3 band and a approximately 68-kd PC2 band. Adrenocorticotrophic hormone-secreting adenomas expressed mainly PC3 as determined by immunoblotting and immunohistochemistry, whereas all other adenoma groups expressed predominantly PC2. These results indicate that the enzymes capable of processing CgA and other members of the Cg/Sg family to peptides with biological activity such as pancreastatin are widely expressed in human pituitary adenomas and in non-neoplastic pituitaries, with adrenocorticotrophic hormone tumors expressing predominantly PC3 and other adenomas expressing mainly PC2. The infrequent expression of CgA protein and pancreastatin peptides in normal and neoplastic prolactin cells suggests a unique role of CgA in these tumors.
Full text
PDF








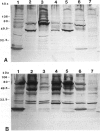
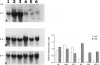
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden S. D., Rutherford N. G., Guest P. C., Curry W. J., Bailyes E. M., Johnston C. F., Hutton J. C. The post-translational processing of chromogranin A in the pancreatic islet: involvement of the eukaryote subtilisin PC2. Biochem J. 1994 Mar 15;298(Pt 3):521–528. doi: 10.1042/bj2980521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. L., Bailyes E. M., Nielsen E., Guest P. C., Rutherford N. G., Arden S. D., Hutton J. C. Identification of the type 2 proinsulin processing endopeptidase as PC2, a member of the eukaryote subtilisin family. J Biol Chem. 1992 Jul 25;267(21):15229–15236. [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994 Jul 29;78(2):263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Cetin Y., Aunis D., Bader M. F., Galindo E., Jörns A., Bargsten G., Grube D. Chromostatin, a chromogranin A-derived bioactive peptide, is present in human pancreatic insulin (beta) cells. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2360–2364. doi: 10.1073/pnas.90.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Deftos L. J., O'Connor D. T., Wilson C. B., Fitzgerald P. A. Human pituitary tumors secrete chromogranin-A. J Clin Endocrinol Metab. 1989 May;68(5):869–872. doi: 10.1210/jcem-68-5-869. [DOI] [PubMed] [Google Scholar]
- Dillen L., Miserez B., Claeys M., Aunis D., De Potter W. Posttranslational processing of proenkephalins and chromogranins/secretogranins. Neurochem Int. 1993 Apr;22(4):315–352. doi: 10.1016/0197-0186(93)90016-x. [DOI] [PubMed] [Google Scholar]
- Egger C., Kirchmair R., Hogue-Angeletti R., Fischer-Colbrie R., Winkler H. Different degrees of processing of secretogranin II in large dense core vesicles of bovine adrenal medulla and sympathetic axons correlate with their content of soluble PC1 and PC2. Neurosci Lett. 1993 Sep 3;159(1-2):199–201. doi: 10.1016/0304-3940(93)90833-7. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Huttner W. B., Mallet J., O'Connor D. T., Winkler H., Zanini A. A nomenclature proposal for the chromogranin/secretogranin proteins. Neuroscience. 1987 Jun;21(3):1019–1021. doi: 10.1016/0306-4522(87)90056-x. [DOI] [PubMed] [Google Scholar]
- Fasciotto B. H., Trauss C. A., Greeley G. H., Cohn D. V. Parastatin (porcine chromogranin A347-419), a novel chromogranin A-derived peptide, inhibits parathyroid cell secretion. Endocrinology. 1993 Aug;133(2):461–466. doi: 10.1210/endo.133.2.8344192. [DOI] [PubMed] [Google Scholar]
- Galindo E., Mendez M., Calvo S., Gonzalez-Garcia C., Ceña V., Hubert P., Bader M. F., Aunis D. Chromostatin receptors control calcium channel activity in adrenal chromaffin cells. J Biol Chem. 1992 Jan 5;267(1):407–412. [PubMed] [Google Scholar]
- Galindo E., Rill A., Bader M. F., Aunis D. Chromostatin, a 20-amino acid peptide derived from chromogranin A, inhibits chromaffin cell secretion. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1426–1430. doi: 10.1073/pnas.88.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G. E., Gabbai F. B., O'Connor D. T., Dinh T. Q., Kennedy B., Ziegler M. G., Takiyyuddin M. A. Does chromostatin influence catecholamine release or blood pressure in vivo? Peptides. 1994 Jan;15(1):195–197. doi: 10.1016/0196-9781(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Koga M., Kouhara H., Arita N., Hayakawa T., Kishimoto T., Sato B. Expression patterns of messenger ribonucleic acids encoding prohormone convertases (PC2 and PC3) in human pituitary adenomas. Clin Endocrinol (Oxf) 1994 Aug;41(2):185–191. doi: 10.1111/j.1365-2265.1994.tb02528.x. [DOI] [PubMed] [Google Scholar]
- Helman L. J., Ahn T. G., Levine M. A., Allison A., Cohen P. S., Cooper M. J., Cohn D. V., Israel M. A. Molecular cloning and primary structure of human chromogranin A (secretory protein I) cDNA. J Biol Chem. 1988 Aug 15;263(23):11559–11563. [PubMed] [Google Scholar]
- Huttner W. B., Gerdes H. H., Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991 Jan;16(1):27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Peshavaria M. Proteolytic processing of chromogranin A in purified insulin granules. Formation of a 20 kDa N-terminal fragment (betagranin) by the concerted action of a Ca2+-dependent endopeptidase and carboxypeptidase H (EC 3.4.17.10). Biochem J. 1987 Jun 1;244(2):457–464. doi: 10.1042/bj2440457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Chandler W. F., Smart J. B., England B. G., Lloyd R. V. Differentiation of human pituitary adenomas determines the pattern of chromogranin/secretogranin messenger ribonucleic acid expression. J Clin Endocrinol Metab. 1993 Mar;76(3):728–735. doi: 10.1210/jcem.76.3.7680355. [DOI] [PubMed] [Google Scholar]
- Kirchmair R., Gee P., Hogue-Angeletti R., Laslop A., Fischer-Colbrie R., Winkler H. Immunological characterization of the endoproteases PC1 and PC2 in adrenal chromaffin granules and in the pituitary gland. FEBS Lett. 1992 Feb 10;297(3):302–305. doi: 10.1016/0014-5793(92)80560-4. [DOI] [PubMed] [Google Scholar]
- Kirchmair R., Gee P., Hogue-Angeletti R., Laslop A., Fischer-Colbrie R., Winkler H. Immunological characterization of the endoproteases PC1 and PC2 in adrenal chromaffin granules and in the pituitary gland. FEBS Lett. 1992 Feb 10;297(3):302–305. doi: 10.1016/0014-5793(92)80560-4. [DOI] [PubMed] [Google Scholar]
- Konecki D. S., Benedum U. M., Gerdes H. H., Huttner W. B. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987 Dec 15;262(35):17026–17030. [PubMed] [Google Scholar]
- Konoshita T., Gasc J. M., Villard E., Takeda R., Seidah N. G., Corvol P., Pinet F. Expression of PC2 and PC1/PC3 in human pheochromocytomas. Mol Cell Endocrinol. 1994 Mar;99(2):307–314. doi: 10.1016/0303-7207(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Iacangelo A., Eiden L. E., Cano M., Jin L., Grimes M. Chromogranin A and B messenger ribonucleic acids in pituitary and other normal and neoplastic human endocrine tissues. Lab Invest. 1989 Apr;60(4):548–556. [PubMed] [Google Scholar]
- Lloyd R. V., Jin L., Kulig E., Fields K. Molecular approaches for the analysis of chromogranins and secretogranins. Diagn Mol Pathol. 1992 Mar;1(1):2–15. doi: 10.1097/00019606-199203000-00002. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Schmidt K., Coleman K., Wilson B. S. Prolactin and growth hormone synthesis and thymidine incorporation in dissociated rat pituitary tumor cells. Proc Soc Exp Biol Med. 1986 Jan;181(1):18–23. doi: 10.3181/00379727-181-42219. [DOI] [PubMed] [Google Scholar]
- McGrath-Linden S. J., Johnston C. F., O'Connor D. T., Shaw C., Buchanan K. D. Pancreastatin-like immunoreactivity in human carcinoid disease. Regul Pept. 1991 Mar 26;33(1):55–70. doi: 10.1016/0167-0115(91)90015-9. [DOI] [PubMed] [Google Scholar]
- Mouland A. J., Bevan S., White J. H., Hendy G. N. Human chromogranin A gene. Molecular cloning, structural analysis, and neuroendocrine cell-specific expression. J Biol Chem. 1994 Mar 4;269(9):6918–6926. [PubMed] [Google Scholar]
- Riva C., Leutner M., Capella C., Usellini L., la Rosa S., Brianza M. R., Buffa R. Different expression of chromogranin A and chromogranin B in various types of pituitary adenomas. Zentralbl Pathol. 1993 Jun;139(2):165–170. [PubMed] [Google Scholar]
- Rosa P., Bassetti M., Weiss U., Huttner W. B. Widespread occurrence of chromogranins/secretogranins in the matrix of secretory granules of endocrinologically silent pituitary adenomas. J Histochem Cytochem. 1992 Apr;40(4):523–533. doi: 10.1177/40.4.1552186. [DOI] [PubMed] [Google Scholar]
- Schmid K. W., Kröll M., Hittmair A., Maier H., Tötsch M., Gasser R., Finkenstett G., Hogue-Angeletti R., Fischer-Colbrie R. Chromogranin A and B in adenomas of the pituitary. An immunohistochemical study of 42 cases. Am J Surg Pathol. 1991 Nov;15(11):1072–1077. doi: 10.1097/00000478-199111000-00006. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Siegel E. G., Lamberts R., Gallwitz B., Creutzfeldt W. Pancreastatin: molecular and immunocytochemical characterization of a novel peptide in porcine and human tissues. Endocrinology. 1988 Sep;123(3):1395–1404. doi: 10.1210/endo-123-3-1395. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Sjöholm A., Funakoshi A., Efendić S., Ostenson C. G., Hellerström C. Long term inhibitory effects of pancreastatin and diazepam binding inhibitor on pancreatic beta-cell deoxyribonucleic acid replication, polyamine content, and insulin secretion. Endocrinology. 1991 Jun;128(6):3277–3282. doi: 10.1210/endo-128-6-3277. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Song J. Y., Jin L., Chandler W. F., England B. G., Smart J. B., Landefeld T. D., Lloyd R. V. Gonadotropin-releasing hormone regulates gonadotropin beta-subunit and chromogranin-B messenger ribonucleic acids in cultured chromogranin-A-positive pituitary adenomas. J Clin Endocrinol Metab. 1990 Sep;71(3):622–630. doi: 10.1210/jcem-71-3-622. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Efendić S., Mutt V., Makk G., Feistner G. J., Barchas J. D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986 Dec 4;324(6096):476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Jeziorowski T., Wuttke W., Grube D. Secretory granules and granins in hyperstimulated male rat gonadotropes. J Histochem Cytochem. 1993 Dec;41(12):1801–1812. doi: 10.1177/41.12.8245429. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Uchiyama Y., Grube D. Topology of chromogranin A and secretogranin II in the rat anterior pituitary: potential marker proteins for distinct secretory pathways in gonadotrophs. Histochemistry. 1991;96(4):285–293. doi: 10.1007/BF00271348. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Huttner W. B. Synaptophysin and chromogranins/secretogranins--widespread constituents of distinct types of neuroendocrine vesicles and new tools in tumor diagnosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;58(2):95–121. doi: 10.1007/BF02890062. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Phan S. H., Lloyd R. V. Chromogranin from normal human adrenal glands: purification by monoclonal antibody affinity chromatography and partial N-terminal amino acid sequence. Regul Pept. 1986 Feb;13(3-4):207–223. doi: 10.1016/0167-0115(86)90040-6. [DOI] [PubMed] [Google Scholar]
- Winkler H., Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992 Aug;49(3):497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Rozansky D. J., Webster N. J., O'Connor D. T. Cell type-specific gene expression in the neuroendocrine system. A neuroendocrine-specific regulatory element in the promoter of chromogranin A, a ubiquitous secretory granule core protein. J Clin Invest. 1994 Jul;94(1):118–129. doi: 10.1172/JCI117297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A., Mains R. E. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem. 1994 Jul 1;269(26):17440–17447. [PubMed] [Google Scholar]