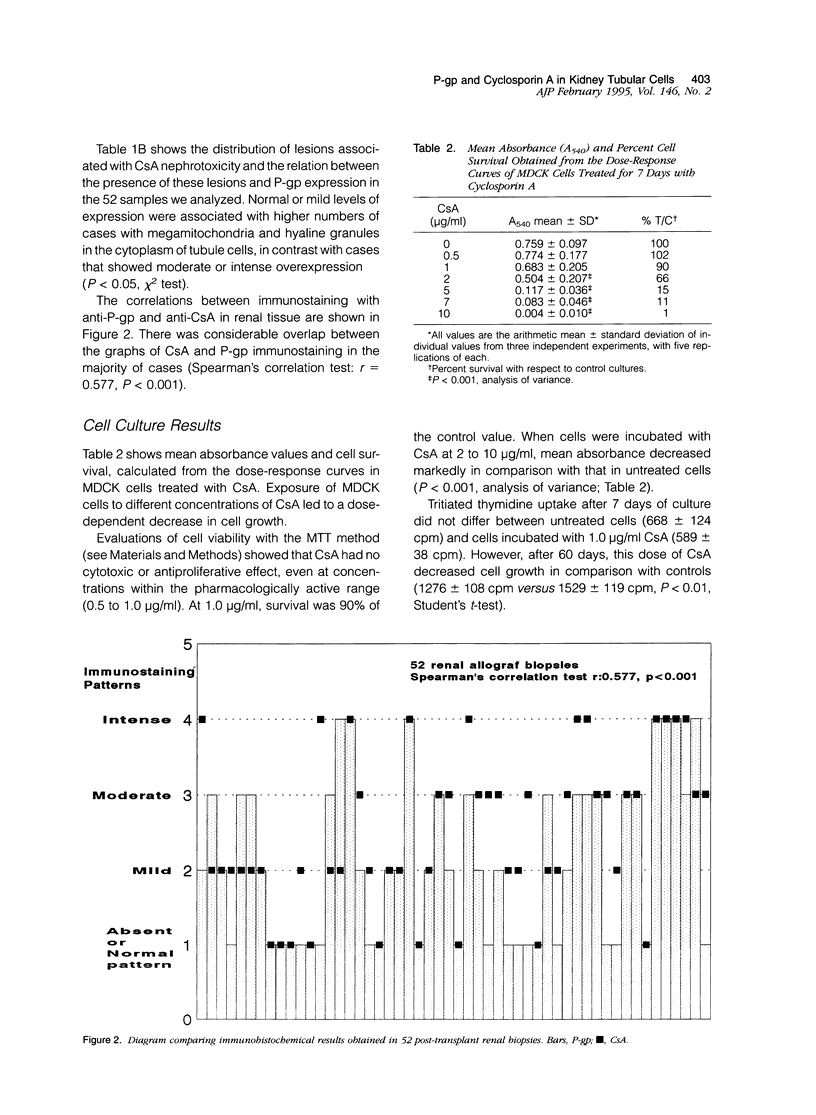
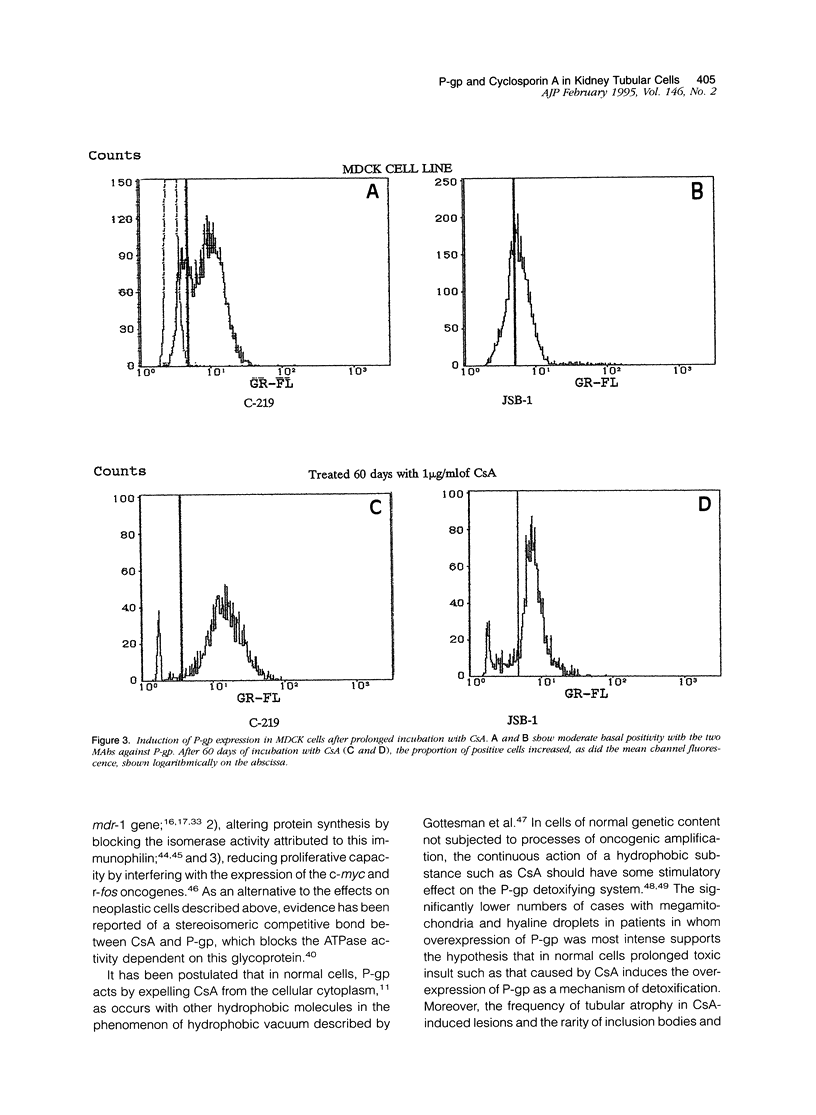
Abstract
P-glycoprotein (P-gp), encoded in humans by the mdr-1 gene, acts physiologically as an efflux pump to expel hydrophobic substances from cells. This glycoprotein is closely related to multidrug resistance in tumor cells and can be modulated by cyclosporin A (CsA). We investigated the relationship between CsA and P-gp in 52 renal allograft biopsies and in cultures of Madin-Darby canine kidney (MDCK) renal tubule cells to determine whether the intrarenal accumulation of CsA or chronic stimulation with the drug modified the expression of P-gp. Expression of P-gp and CsA was analyzed by immunohistochemistry. Immunostaining was evaluated semiquantitatively. Modulation of P-gp in MDCK cells after chronic stimulation with CsA for 7, 30, and 60 days was analyzed by flow cytometry. P-gp and CsA immunostaining in renal post-transplant biopsies showed considerable overlap in all cases (Spearman's test, r = 0.577, P < 0.001). After 7 days in vitro, the number of cells expressing P-gp increased progressively; a further increase in mean fluorescence was found after 60 days (P < 0.001, Student's t-test). Our findings suggest that in non-neoplastic cells, CsA may stimulate P-gp as a mechanism of detoxification. Individual differences in the adaptive responses to glycoprotein may be responsible for the appearance of nephrotoxicity or a CsA-resistant rejection reaction in cases of overexpression on lymphocytes and macrophages.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberg D. G., Schreiber S. L. Structure-based design of a cyclophilin-calcineurin bridging ligand. Science. 1993 Oct 8;262(5131):248–250. doi: 10.1126/science.8211144. [DOI] [PubMed] [Google Scholar]
- Almond P. S., Gillingham K. J., Sibley R., Moss A., Melin M., Leventhal J., Manivel C., Kyriakides P., Payne W. D., Dunn D. L. Renal transplant function after ten years of cyclosporine. Transplantation. 1992 Feb;53(2):316–323. doi: 10.1097/00007890-199202010-00012. [DOI] [PubMed] [Google Scholar]
- Charuk J. H., Reithmeier R. A. Interaction of P-glycoprotein with a hydrophobic component of rat urine. Biochem Biophys Res Commun. 1992 Jul 31;186(2):796–802. doi: 10.1016/0006-291x(92)90816-4. [DOI] [PubMed] [Google Scholar]
- Chaudhary P. M., Roninson I. B. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J Natl Cancer Inst. 1993 Apr 21;85(8):632–639. doi: 10.1093/jnci/85.8.632. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Boccia J., Casals D., Bertino J. R., Melamed M. R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990 Sep;38(9):1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Daidone M. G., Silvestrini R., Sanfilippo O., Zaffaroni N., Varini M., De Lena M. Reliability of an in vitro short-term assay to predict the drug sensitivity of human breast cancer. Cancer. 1985 Aug 1;56(3):450–456. doi: 10.1002/1097-0142(19850801)56:3<450::aid-cncr2820560306>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Efferth T., Volm M. Modulation of P-glycoprotein-mediated multidrug resistance by monoclonal antibodies, immunotoxins or antisense oligodeoxynucleotides in kidney carcinoma and normal kidney cells. Oncology. 1993 Jul-Aug;50(4):303–308. doi: 10.1159/000227200. [DOI] [PubMed] [Google Scholar]
- Erlichman C., Moore M., Thiessen J. J., Kerr I. G., Walker S., Goodman P., Bjarnason G., DeAngelis C., Bunting P. Phase I pharmacokinetic study of cyclosporin A combined with doxorubicin. Cancer Res. 1993 Oct 15;53(20):4837–4842. [PubMed] [Google Scholar]
- Fathman C. G., Myers B. D. Cyclosporine therapy for autoimmune disease. N Engl J Med. 1992 Jun 18;326(25):1693–1695. doi: 10.1056/NEJM199206183262509. [DOI] [PubMed] [Google Scholar]
- Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993 Sep 15;216(3):689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- García Del Moral R., Navarro N., Montes A., Aguilar D., Aneiros J. Immunohistochemical markers of renal tubular injury and cyclosporin nephrotoxicity in kidney allograft biopsies. Nephron. 1992;62(1):121–122. doi: 10.1159/000187017. [DOI] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993 Feb 15;53(4):747–754. [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Herweijer H., Sonneveld P., Baas F., Nooter K. Expression of mdr1 and mdr3 multidrug-resistance genes in human acute and chronic leukemias and association with stimulation of drug accumulation by cyclosporine. J Natl Cancer Inst. 1990 Jul 4;82(13):1133–1140. doi: 10.1093/jnci/82.13.1133. [DOI] [PubMed] [Google Scholar]
- Herzog C. E., Tsokos M., Bates S. E., Fojo A. T. Increased mdr-1/P-glycoprotein expression after treatment of human colon carcinoma cells with P-glycoprotein antagonists. J Biol Chem. 1993 Feb 5;268(4):2946–2952. [PubMed] [Google Scholar]
- Hsu V. L., Heald S. L., Harding M. W., Handschumacher R. E., Armitage I. M. Structural elements pertinent to the interaction of cyclosporin A with its specific receptor protein, cyclophilin. Biochem Pharmacol. 1990 Jul 1;40(1):131–140. doi: 10.1016/0006-2952(90)90188-q. [DOI] [PubMed] [Google Scholar]
- Isoniemi H. M., Krogerus L., von Willebrand E., Taskinen E., Ahonen J., Häyry P. Histopathological findings in well-functioning, long-term renal allografts. Kidney Int. 1992 Jan;41(1):155–160. doi: 10.1038/ki.1992.21. [DOI] [PubMed] [Google Scholar]
- Kahan B. D. Optimization of cyclosporine therapy. Transplant Proc. 1993 Aug;25(4 Suppl 3):5–9. [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kemnitz J., Uysal A., Haverich A., Heublein B., Cohnert T. R., Stangel W., Georgii A. Multidrug resistance in heart transplant patients: a preliminary communication on a possible mechanism of therapy-resistant rejection. J Heart Lung Transplant. 1991 Mar-Apr;10(2):201–210. [PubMed] [Google Scholar]
- Kute T. E., Quadri Y. Measurement of proliferation nuclear and membrane markers in tumor cells by flow cytometry. J Histochem Cytochem. 1991 Aug;39(8):1125–1130. doi: 10.1177/39.8.1856460. [DOI] [PubMed] [Google Scholar]
- Lemaire M., Fahr A., Maurer G. Pharmacokinetics of cyclosporine: inter- and intra-individual variations and metabolic pathways. Transplant Proc. 1990 Jun;22(3):1110–1112. [PubMed] [Google Scholar]
- Lewis R. M., Podbielski J., Munsell M., Katz S., Van Buren C., Kerman R., Kahan B. Optimization and long-term evaluation of renal function in sandimmune-treated renal allograft recipients. Transplant Proc. 1993 Aug;25(4 Suppl 3):10–12. [PubMed] [Google Scholar]
- Linsenmeyer M. E., Jefferson S., Wolf M., Matthews J. P., Board P. G., Woodcock D. M. Levels of expression of the mdr1 gene and glutathione S-transferase genes 2 and 3 and response to chemotherapy in multiple myeloma. Br J Cancer. 1992 Mar;65(3):471–475. doi: 10.1038/bjc.1992.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath T., Center M. S. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988 Jul 15;48(14):3959–3963. [PubMed] [Google Scholar]
- Mihatsch M. J., Antonovych T., Bohman S. O., Habib R., Helmchen U., Noel L. H., Olsen S., Sibley R. K., Kemény E., Feutren G. Cyclosporin A nephropathy: standardization of the evaluation of kidney biopsies. Clin Nephrol. 1994 Jan;41(1):23–32. [PubMed] [Google Scholar]
- Mihatsch M. J., Thiel G., Ryffel B. Histopathology of cyclosporine nephrotoxicity. Transplant Proc. 1988 Jun;20(3 Suppl 3):759–771. [PubMed] [Google Scholar]
- Nooter K., Herweijer H. Multidrug resistance (mdr) genes in human cancer. Br J Cancer. 1991 May;63(5):663–669. doi: 10.1038/bjc.1991.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Gottesman M. M., Ueda K., Lovelace E., Rutherford A. V., Willingham M. C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4486–4490. doi: 10.1073/pnas.85.12.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileri S. A., Sabattini E., Falini B., Tazzari P. L., Gherlinzoni F., Michieli M. G., Damiani D., Zucchini L., Gobbi M., Tsuruo T. Immunohistochemical detection of the multidrug transport protein P170 in human normal tissues and malignant lymphomas. Histopathology. 1991 Aug;19(2):131–140. doi: 10.1111/j.1365-2559.1991.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Milroy R., Kaye S. B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989 Aug 15;49(16):4435–4440. [PubMed] [Google Scholar]
- Reed J. C., Nowell P. C., Hoover R. G. Regulation of c-myc mRNA levels in normal human lymphocytes by modulators of cell proliferation. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4221–4224. doi: 10.1073/pnas.82.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson I. B. The role of the MDR1 (P-glycoprotein) gene in multidrug resistance in vitro and in vivo. Biochem Pharmacol. 1992 Jan 9;43(1):95–102. doi: 10.1016/0006-2952(92)90666-7. [DOI] [PubMed] [Google Scholar]
- Ryffel B., Woerly G., Rodriguez C., Foxwell B. M. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for cyclosporine. J Recept Res. 1991;11(1-4):675–686. doi: 10.3109/10799899109066435. [DOI] [PubMed] [Google Scholar]
- Saeki T., Ueda K., Tanigawara Y., Hori R., Komano T. P-glycoprotein-mediated transcellular transport of MDR-reversing agents. FEBS Lett. 1993 Jun 7;324(1):99–102. doi: 10.1016/0014-5793(93)81540-g. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Scoble J. E., Senior J. C., Chan P., Varghese Z., Sweny P., Moorhead J. F. In vitro cyclosporine toxicity. The effect of verapamil. Transplantation. 1989 Apr;47(4):647–650. doi: 10.1097/00007890-198904000-00016. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Schindler M. Cell biological mechanisms of multidrug resistance in tumors. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3497–3504. doi: 10.1073/pnas.91.9.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solez K., Axelsen R. A., Benediktsson H., Burdick J. F., Cohen A. H., Colvin R. B., Croker B. P., Droz D., Dunnill M. S., Halloran P. F. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993 Aug;44(2):411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Immunohistochemical localization in normal tissues of different epitopes in the multidrug transport protein P170: evidence for localization in brain capillaries and crossreactivity of one antibody with a muscle protein. J Histochem Cytochem. 1989 Feb;37(2):159–164. doi: 10.1177/37.2.2463300. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Cyclosporins as drug resistance modifiers. Biochem Pharmacol. 1992 Jan 9;43(1):109–117. doi: 10.1016/0006-2952(92)90668-9. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Wright K. A., Wallace H. M. Effects of cyclosporin A and a non-immunosuppressive analogue, O-acetyl cyclosporin A, upon the growth of parent and multidrug resistant human lung cancer cells in vitro. Br J Cancer. 1992 Mar;65(3):335–340. doi: 10.1038/bjc.1992.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J. L., Szabo G., Jr, Pine P. S., Gottesman M. M., Goldenberg S., Aszalos A. The effect of ion channel blockers, immunosuppressive agents, and other drugs on the activity of the multi-drug transporter. Int J Cancer. 1993 May 28;54(3):456–461. doi: 10.1002/ijc.2910540317. [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Hartz P. A. Mechanisms of cyclosporine A toxicity in defined cultures of renal tubule epithelia: a role for cysteine proteases. Cell Biol Int Rep. 1991 Dec;15(12):1243–1258. doi: 10.1016/0309-1651(91)90096-2. [DOI] [PubMed] [Google Scholar]
- Wright K. A., Twentyman P. R. Derivation and characterisation of a mouse tumour cell line with acquired resistance to cyclosporin A. Eur J Cancer. 1993;29A(3):389–394. doi: 10.1016/0959-8049(93)90393-t. [DOI] [PubMed] [Google Scholar]
- Wu L., Smythe A. M., Stinson S. F., Mullendore L. A., Monks A., Scudiero D. A., Paull K. D., Koutsoukos A. D., Rubinstein L. V., Boyd M. R. Multidrug-resistant phenotype of disease-oriented panels of human tumor cell lines used for anticancer drug screening. Cancer Res. 1992 Jun 1;52(11):3029–3034. [PubMed] [Google Scholar]
- Yousem S. A., Sartori D., Sonmez-Alpan E. Multidrug resistance in lung allograft recipients: possible correlation with the development of acute and chronic rejection. J Heart Lung Transplant. 1993 Jan-Feb;12(1 Pt 1):20–26. [PubMed] [Google Scholar]