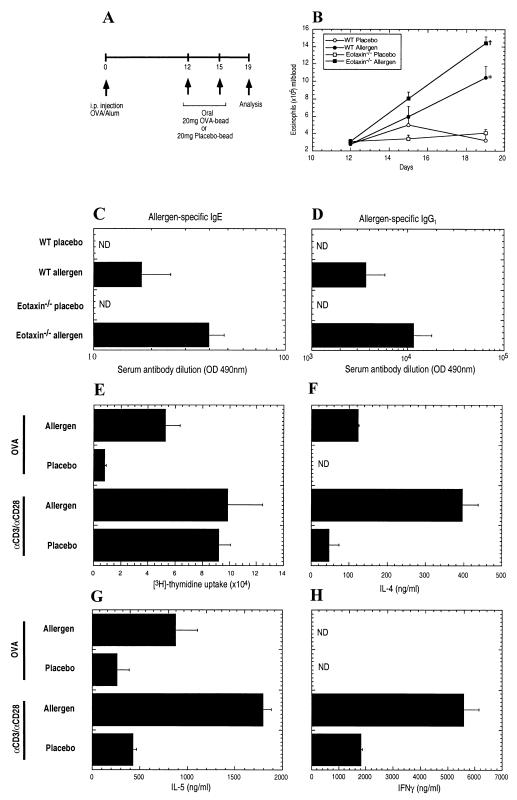
Figure 1.
Characterization of gastrointestinal allergy in wild-type and eotaxin-deficient mice. (A) Experimental regime. Mice were i.p. injected with 50 μg OVA/1 mg alum on day 0. On days 12 and 15, mice were orally administered 20 mg of encapsulated placebo- and OVA-enteric coated beads followed by acidified H2O (pH 2.0). Four days after the last challenge, mice were killed and parameters measured. (B) Eosinophil numbers in the peripheral blood of placebo- and oral-allergen-challenged wild-type (WT) and eotaxin-deficient (eotaxin−/−) mice. Inset specifies the groups. (C and D) Levels of allergen-specific IgE (C) and IgG1 (D) in the serum of placebo- and oral allergen-challenged wild-type (WT) and eotaxin-deficient (eotaxin−/−) mice were determined by ELISA. (E) Proliferation of splenic T cells stimulated by αCD3/αCD28 or OVA were measured by [3H]-thymidine incorporation. (F–H) Secretion of IL-4 (F), IL-5 (G), and IFNγ (H) by αCD3/αCD28- and OVA-stimulated splenic T cells. Data in B and D represent the mean ± SEM for groups of four to five animals in triplicate experiments and (E–H) mean ± SEM from three individual cultures obtained from four to five mice in each group. Statistical significance of experimental groups was analyzed by using the unpaired Student's t test (B). *, P < 0.05 compared with day 19 allergen challenge of wild-type mice; +, P < 0.001 compared with placebo challenge of wild-type mice on day 19. Unless otherwise indicated, data were obtained on day 19. ND, not detected.