Abstract
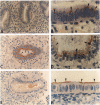
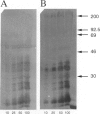
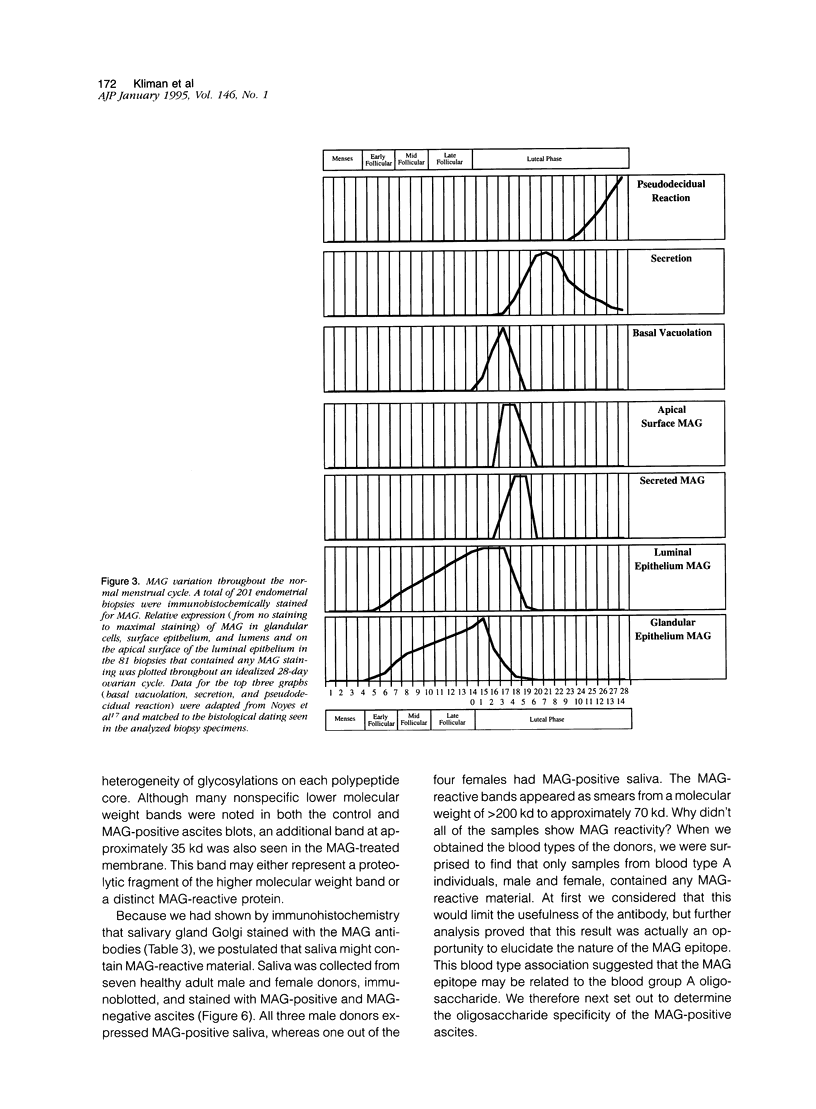
Human endometrial glands synthesize and secrete a high molecular weight mucin-like glycoprotein in a menstrual cycle-dependent fashion. A novel moiety within this Golgi-associated glycoprotein is strongly reactive with IgG antibodies in numerous murine ascites, and has been termed MAG (mouse ascites Golgi). Immunohistochemical staining of 201 endometrial biopsies revealed the following patterns: MAG first appeared in the Golgi on cycle day 5, peaked on day 15, was present on the surface of the luminal epithelium between days 17 and 19, and was no longer detectable after day 19. MAG was also present in cervical, prostate, seminal vesicle, and lacrimal glands, pancreatic acinar cells, gall bladder and bile duct epithelium, and certain cells of the salivary and sweat glands. Interestingly, only tissues from blood group A individuals exhibited this staining. As a common link among all these cell types is the expression of mucins, we speculated that the MAG epitope could be a mucin-associated blood group A-related epitope. This hypothesis was tested by absorption experiments with a variety of glycoconjugates and erythrocytes and by immunoblots of MAG-rich material. The absorption studies demonstrated that only type III porcine mucin (< 1% sialic acid) and blood type A or AB erythrocytes were able to absorb the anti-MAG antibody. Inasmuch as N-acetyl-galactosamine alone, the terminal blood group A carbohydrate, did not block MAG antibody binding, the MAG epitope appears to involve N-acetylgalactosamine plus other determinants. Immunoblots of endometrial extracts and saliva from blood type A individuals revealed MAG-reactive material with a molecular weight > 200 kd under reducing conditions. Because the MAG epitope appears on the endometrial surface during the purported implantation window, we speculate that mucin-like epitopes could play a role in the earliest apposition phases of conceptus-endometrial interaction.
Full text
PDF





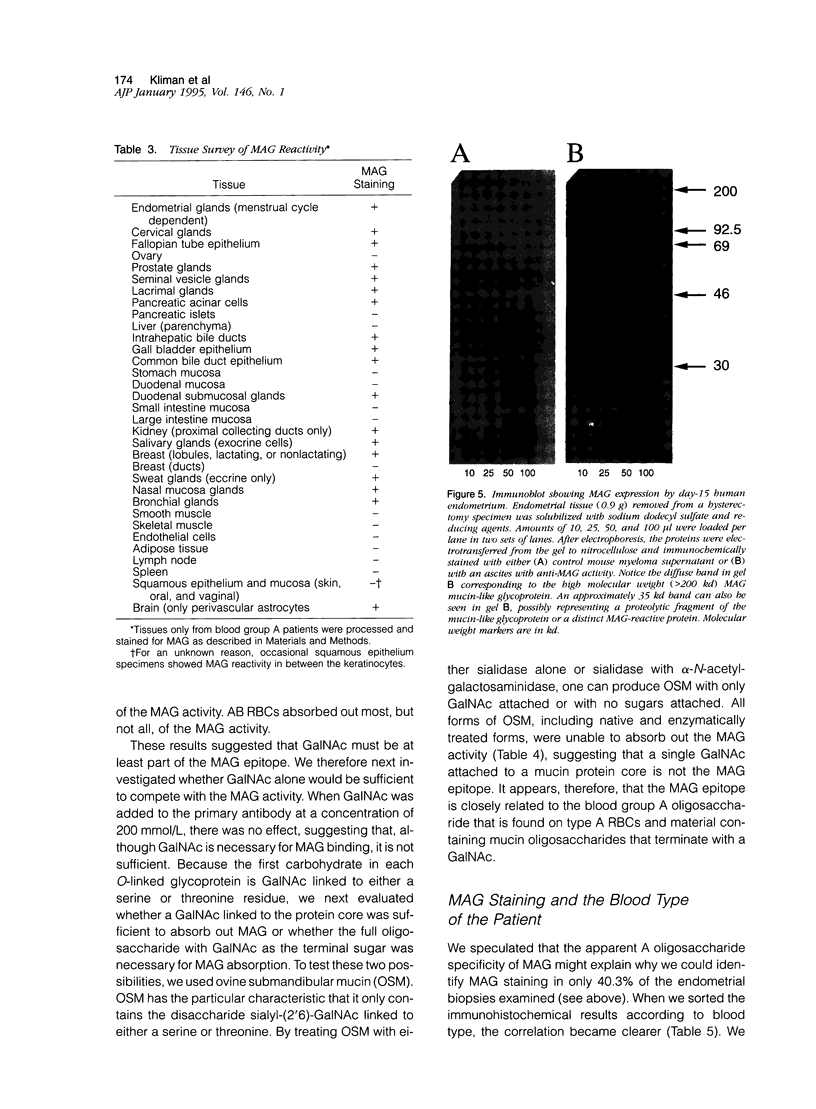
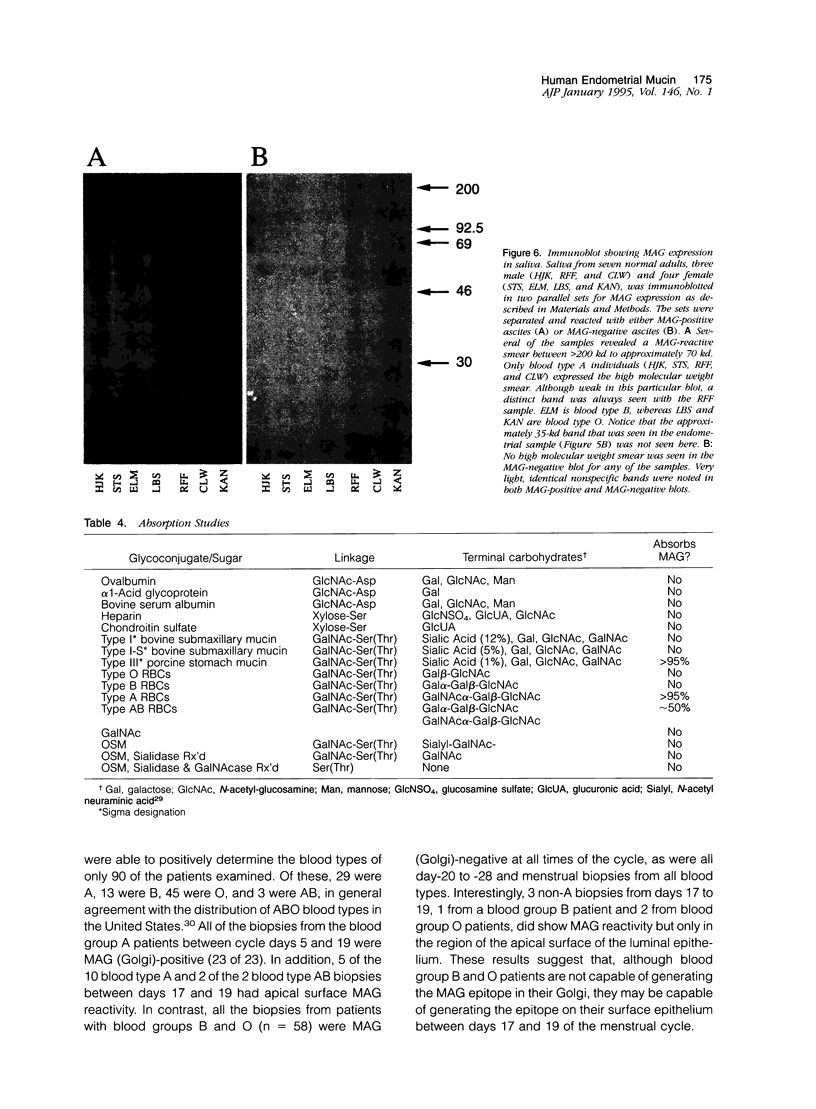






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. L., Olson G. E., Hoffman L. H. Stage-specific alterations in the apical membrane glycoproteins of endometrial epithelial cells related to implantation in rabbits. Biol Reprod. 1986 May;34(4):701–720. doi: 10.1095/biolreprod34.4.701. [DOI] [PubMed] [Google Scholar]
- Aplin J. D. Glycans as biochemical markers of human endometrial secretory differentiation. J Reprod Fertil. 1991 Jul;92(2):525–541. doi: 10.1530/jrf.0.0920525. [DOI] [PubMed] [Google Scholar]
- Aplin J. D. Glycans as biochemical markers of human endometrial secretory differentiation. J Reprod Fertil. 1991 Jul;92(2):525–541. doi: 10.1530/jrf.0.0920525. [DOI] [PubMed] [Google Scholar]
- Axiotis C. A., Guarch R., Merino M. J., Laporte N., Neumann R. D. P-glycoprotein expression is increased in human secretory and gestational endometrium. Lab Invest. 1991 Nov;65(5):577–581. [PubMed] [Google Scholar]
- Axiotis C. A., Monteagudo C., Merino M. J., LaPorte N., Neumann R. D. Immunohistochemical detection of P-glycoprotein in endometrial adenocarcinoma. Am J Pathol. 1991 Apr;138(4):799–806. [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Found Symp. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- Carson D. D., Farrar J. D., Laidlaw J., Wright D. A. Selective activation of the N-glycosylation apparatus in uteri by estrogen. J Biol Chem. 1990 Feb 15;265(5):2947–2955. [PubMed] [Google Scholar]
- Chávez D. J., Anderson T. L. The glycocalyx of the mouse uterine luminal epithelium during estrus, early pregnancy, the peri-implantation period, and delayed implantation. I. Acquisition of Ricinus communis I binding sites during pregnancy. Biol Reprod. 1985 Jun;32(5):1135–1142. doi: 10.1095/biolreprod32.5.1135. [DOI] [PubMed] [Google Scholar]
- Devine P. L., McKenzie I. F. Mucins: structure, function, and associations with malignancy. Bioessays. 1992 Sep;14(9):619–625. doi: 10.1002/bies.950140909. [DOI] [PubMed] [Google Scholar]
- Ellis I. O., Robins R. A., Elston C. W., Blamey R. W., Ferry B., Baldwin R. W. A monoclonal antibody, NCRC-11, raised to human breast carcinoma. 1. Production and immunohistological characterization. Histopathology. 1984 May;8(3):501–516. doi: 10.1111/j.1365-2559.1984.tb02360.x. [DOI] [PubMed] [Google Scholar]
- Enders A. C., Schlafke S. Surface coats of the mouse blastocyst and uterus during the preimplantation period. Anat Rec. 1974 Sep;180(1):31–45. doi: 10.1002/ar.1091800105. [DOI] [PubMed] [Google Scholar]
- Fay T. N., Grudzinskas J. G. Human endometrial peptides: a review of their potential role in implantation and placentation. Hum Reprod. 1991 Oct;6(9):1311–1326. doi: 10.1093/oxfordjournals.humrep.a137533. [DOI] [PubMed] [Google Scholar]
- Feinberg R. F., Kliman H. J., Lockwood C. J. Is oncofetal fibronectin a trophoblast glue for human implantation? Am J Pathol. 1991 Mar;138(3):537–543. [PMC free article] [PubMed] [Google Scholar]
- Finstad C. L., Saigo P. E., Rubin S. C., Federici M. G., Provencher D. M., Hoskins W. J., Lewis J. L., Jr, Lloyd K. O. Immunohistochemical localization of P-glycoprotein in adult human ovary and female genital tract of patients with benign gynecological conditions. J Histochem Cytochem. 1990 Nov;38(11):1677–1681. doi: 10.1177/38.11.1976674. [DOI] [PubMed] [Google Scholar]
- Finstad C. L., Yin B. W., Gordon C. M., Federici M. G., Welt S., Lloyd K. O. Some monoclonal antibody reagents (C219 and JSB-1) to P-glycoprotein contain antibodies to blood group A carbohydrate determinants: a problem of quality control for immunohistochemical analysis. J Histochem Cytochem. 1991 Dec;39(12):1603–1610. doi: 10.1177/39.12.1682363. [DOI] [PubMed] [Google Scholar]
- GRAHAM E. R., GOTTSCHALK A. Studies on mucoproteins. I. The structure of the prosthetic group of ovine submaxillary gland mucoprotein. Biochim Biophys Acta. 1960 Mar 11;38:513–524. doi: 10.1016/0006-3002(60)91286-5. [DOI] [PubMed] [Google Scholar]
- Gooi H. C., Feizi T. Natural antibodies as contaminants of hybridoma products. Biochem Biophys Res Commun. 1982 May 31;106(2):539–545. doi: 10.1016/0006-291x(82)91144-5. [DOI] [PubMed] [Google Scholar]
- Gottschalk A., Schauer H., Uhlenbruck G. Immunological properties of ovine submaxillary glycoprotein. Hoppe Seylers Z Physiol Chem. 1971 Feb;352(2):117–124. doi: 10.1515/bchm2.1971.352.1.117. [DOI] [PubMed] [Google Scholar]
- Harper M. J. The implantation window. Baillieres Clin Obstet Gynaecol. 1992 Jun;6(2):351–371. doi: 10.1016/s0950-3552(05)80092-6. [DOI] [PubMed] [Google Scholar]
- Hewitt K., Beer A. E., Grinnell F. Disappearance of anionic sites from the surface of the rat endometrial epithelium at the time of blastocyst implantation. Biol Reprod. 1979 Oct;21(3):691–707. doi: 10.1095/biolreprod21.3.691. [DOI] [PubMed] [Google Scholar]
- Hilkens J., Ligtenberg M. J., Vos H. L., Litvinov S. V. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992 Sep;17(9):359–363. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- Ii M., Kurata H., Itoh N., Yamashina I., Kawasaki T. Molecular cloning and sequence analysis of cDNA encoding the macrophage lectin specific for galactose and N-acetylgalactosamine. J Biol Chem. 1990 Jul 5;265(19):11295–11298. [PubMed] [Google Scholar]
- Kimber S. J., Lindenberg S. Hormonal control of a carbohydrate epitope involved in implantation in mice. J Reprod Fertil. 1990 May;89(1):13–21. doi: 10.1530/jrf.0.0890013. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Dowbenko D., Imai Y., Henzel W. J., Grimley C., Fennie C., Gillett N., Watson S. R., Rosen S. D. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992 Jun 12;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Lessey B. A., Castelbaum A. J., Buck C. A., Lei Y., Yowell C. W., Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril. 1994 Sep;62(3):497–506. [PubMed] [Google Scholar]
- Lessey B. A., Damjanovich L., Coutifaris C., Castelbaum A., Albelda S. M., Buck C. A. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992 Jul;90(1):188–195. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey B. A., Pindzola J. A. Tumor-associated glycoprotein (TAG-72) in endometriotic implants. J Clin Endocrinol Metab. 1993 Apr;76(4):1075–1079. doi: 10.1210/jcem.76.4.7682561. [DOI] [PubMed] [Google Scholar]
- Lindenberg S. Experimental studies on the initial trophoblast endometrial interaction. Dan Med Bull. 1991 Oct;38(5):371–380. [PubMed] [Google Scholar]
- Lindenberg S., Sundberg K., Kimber S. J., Lundblad A. The milk oligosaccharide, lacto-N-fucopentaose I, inhibits attachment of mouse blastocysts on endometrial monolayers. J Reprod Fertil. 1988 May;83(1):149–158. doi: 10.1530/jrf.0.0830149. [DOI] [PubMed] [Google Scholar]
- Lindenberg S. Ultrastructure in human implantation: transmission and scanning electron microscopy. Baillieres Clin Obstet Gynaecol. 1991 Mar;5(1):1–14. doi: 10.1016/s0950-3552(05)80067-7. [DOI] [PubMed] [Google Scholar]
- Lopez L. C., Youakim A., Evans S. C., Shur B. D. Evidence for a molecular distinction between Golgi and cell surface forms of beta 1,4-galactosyltransferase. J Biol Chem. 1991 Aug 25;266(24):15984–15991. [PubMed] [Google Scholar]
- Mani S. K., Carson D. D., Glasser S. R. Steroid hormones differentially modulate glycoconjugate synthesis and vectorial secretion by polarized uterine epithelial cells in vitro. Endocrinology. 1992 Jan;130(1):240–248. doi: 10.1210/endo.130.1.1727700. [DOI] [PubMed] [Google Scholar]
- Miller D. J., Macek M. B., Shur B. D. Complementarity between sperm surface beta-1,4-galactosyltransferase and egg-coat ZP3 mediates sperm-egg binding. Nature. 1992 Jun 18;357(6379):589–593. doi: 10.1038/357589a0. [DOI] [PubMed] [Google Scholar]
- Munakata H., Isemura M., Yosizawa Z. Enzymatic sulfation of exogenous high molecular weight glycopeptides by microsomal fraction of the rabbit uterine endometrium. J Biol Chem. 1985 Jun 10;260(11):6851–6856. [PubMed] [Google Scholar]
- Navot D., Bergh P. A., Williams M., Garrisi G. J., Guzman I., Sandler B., Fox J., Schreiner-Engel P., Hofmann G. E., Grunfeld L. An insight into early reproductive processes through the in vivo model of ovum donation. J Clin Endocrinol Metab. 1991 Feb;72(2):408–414. doi: 10.1210/jcem-72-2-408. [DOI] [PubMed] [Google Scholar]
- Navot D., Scott R. T., Droesch K., Veeck L. L., Liu H. C., Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril. 1991 Jan;55(1):114–118. doi: 10.1016/s0015-0282(16)54069-2. [DOI] [PubMed] [Google Scholar]
- Patt L. M., Grimes W. J. Cell surface glycolipid and glycoprotein glycosyltransferases of normal and transformed cells. J Biol Chem. 1974 Jul 10;249(13):4157–4165. [PubMed] [Google Scholar]
- Ravn V., Teglbjaerg C. S., Visfeldt J., Bock J. E., Sørensen H., Dabelsteen E. Mucin-type carbohydrates (type 3 chain antigens) in normal cycling human endometrium. Int J Gynecol Pathol. 1992;11(1):38–46. doi: 10.1097/00004347-199201000-00007. [DOI] [PubMed] [Google Scholar]
- Rose M. C. Mucins: structure, function, and role in pulmonary diseases. Am J Physiol. 1992 Oct;263(4 Pt 1):L413–L429. doi: 10.1152/ajplung.1992.263.4.L413. [DOI] [PubMed] [Google Scholar]
- Roth J., Greenwell P., Watkins W. M. Immunolocalization of blood group A gene specified alpha 1,3N-acetylgalactosaminyltransferase and blood group A substance in the trans-tubular network of the Golgi apparatus and mucus of intestinal goblet cells. Eur J Cell Biol. 1988 Apr;46(1):105–112. [PubMed] [Google Scholar]
- Rye P. D., Bell S. C., Walker R. A. Immunohistochemical expression of tumour-associated glycoprotein and polymorphic epithelial mucin in the human endometrium during the menstrual cycle. J Reprod Fertil. 1993 Mar;97(2):551–556. doi: 10.1530/jrf.0.0970551. [DOI] [PubMed] [Google Scholar]
- Sato M., Muramatsu T., Berger E. G. Immunological detection of cell surface galactosyltransferase in preimplantation mouse embryos. Dev Biol. 1984 Apr;102(2):514–518. doi: 10.1016/0012-1606(84)90219-7. [DOI] [PubMed] [Google Scholar]
- Schneider J., Efferth T., Centeno M. M., Mattern J., Rodríguez-Escudero F. J., Volm M. High rate of expression of multidrug resistance-associated P-glycoprotein in human endometrial carcinoma and normal endometrial tissue. Eur J Cancer. 1993;29A(4):554–558. doi: 10.1016/s0959-8049(05)80150-9. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S. Cell adhesion. Mucins in the mainstream. Nature. 1993 Dec 16;366(6456):630–631. doi: 10.1038/366630a0. [DOI] [PubMed] [Google Scholar]
- Smith Z. D., D'Eugenio-Gumkowski F., Yanagisawa K., Jamieson J. D. Endogenous and monoclonal antibodies to the rat pancreatic acinar cell Golgi complex. J Cell Biol. 1984 Jun;98(6):2035–2046. doi: 10.1083/jcb.98.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27(1-2):57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Thor A., Viglione M. J., Muraro R., Ohuchi N., Schlom J., Gorstein F. Monoclonal antibody B72.3 reactivity with human endometrium: a study of normal and malignant tissues. Int J Gynecol Pathol. 1987;6(3):235–247. doi: 10.1097/00004347-198709000-00005. [DOI] [PubMed] [Google Scholar]
- Vanderhyden B. C., Armstrong D. T. Decreased embryonic survival of in-vitro fertilized oocytes in rats is due to retardation of preimplantation development. J Reprod Fertil. 1988 Jul;83(2):851–857. doi: 10.1530/jrf.0.0830851. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Coon J. S., Dominquez J. M., Jakate S. M., Lebovitz M. D., Chang M. A., Kluskens L. F. Correlation between ABO blood type and Golgi P-glycoprotein expression in epithelia. Lancet. 1990 Jul 7;336(8706):54–55. doi: 10.1016/0140-6736(90)91568-u. [DOI] [PubMed] [Google Scholar]
- Weinstein R. S., Kuszak J. R., Jakate S. M., Lebovitz M. D., Kluskens L. F., Coon J. S. ABO blood type predicts the cytolocalization of anti-P-glycoprotein monoclonal antibody reactivity in human colon and ureter. Hum Pathol. 1990 Sep;21(9):949–958. doi: 10.1016/0046-8177(90)90180-d. [DOI] [PubMed] [Google Scholar]
- Weintraub J., Bischof P., Tseng L., Redard M., Vassilakos P. CA 125 is an excretory product of human endometrial glands. Biol Reprod. 1990 Apr;42(4):721–726. doi: 10.1095/biolreprod42.4.721. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Cellular interactions. Out of equilibrium. Nature. 1991 Aug 8;352(6335):473–474. doi: 10.1038/352473a0. [DOI] [PubMed] [Google Scholar]
- Zalc B., Collet A., Monge M., Ollier-Hartmann M. P., Jacque C., Hartmann L., Baumann N. A. Tamm-Horsfall protein, a kidney marker is expressed on brain sulfogalactosylceramide-positive astroglial structures. Brain Res. 1984 Jan 16;291(1):182–187. doi: 10.1016/0006-8993(84)90669-3. [DOI] [PubMed] [Google Scholar]
- Zeimet A. G., Müller-Holzner E., Marth C., Daxenbichler G., Dapunt O. Tumor marker CA-125 in tissues of the female reproductive tract and in serum during the normal menstrual cycle. Fertil Steril. 1993 May;59(5):1028–1035. doi: 10.1016/s0015-0282(16)55923-8. [DOI] [PubMed] [Google Scholar]