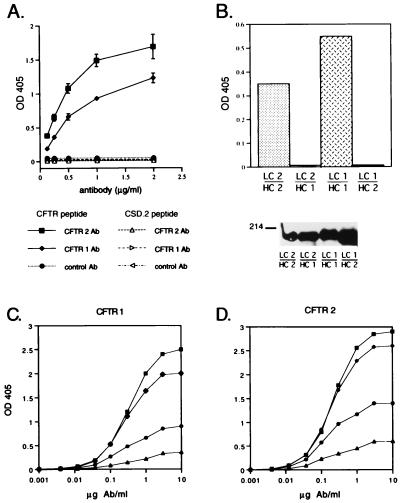
Figure 3.
Assays demonstrating the specificity of the chicken-derived CFTR antibodies. (A) The ability of the CFTR 1 and CFTR 2 chimeric antibodies to bind the CFTR peptide and the unrelated peptide CSD.2 was assayed by ELISA (13). Filled symbols indicate the data obtained on ELISA plates coated with CFTR peptide; open symbols indicate the data obtained on the plates coated with the CSD.2 peptide. Each data point is the mean of six observations. (B) Determination of the specificity of chimeric antibodies comprised of different combinations of the heavy and light chains from the original chicken CFTR antibodies. (Upper) In an ELISA assay, wells coated with CFTR peptide were incubated with the antibodies secreted from cells transfected with each of the four combinations of the plasmids encoding CFTR 1 or CFTR 2 light chain and heavy chain. Supernatants from three independent passages of the cells were tested for binding to the CFTR peptide; the average OD405 is indicated by the bar. LC 1/HC 1, cells were transfected with the light chain of CFTR 1 and the heavy chain of CFTR 1; LC 1/HC 2, cells were transfected with the light chain of CFTR 1 and the heavy chain of CFTR 2, and so forth. (Lower) The supernatants tested in the upper panel were assayed by immunoblot to quantitate the amount of antibody present. Equal volumes of tissue culture supernatant from each transfected cell line were analyzed by electrophoresis on a 10% nondenaturing polyacrylamide gel, transferred to nitrocellulose, and probed with anti-chicken IgG(H+L). (C and D) Experimental dose–response curves of CFTR 1 and CFTR 2 antibodies at four antigen coating concentrations. The four CFTR–BSA coating concentrations were: 0.2 μg/ml (▴), 0.4 μg/ml (•), 0.8 μg/ml (♦), and 1.6 μg/ml (▪). The data points were used to calculate the functional affinity constant of each antibody according to published formulae (28, 29).