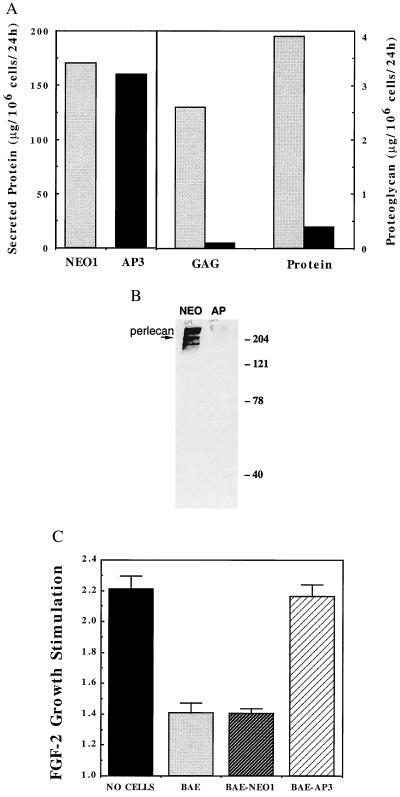
Figure 2.
BAE-AP3 shows significantly reduced proteoglycan synthesis and is less able to inhibit FGF-2 stimulation of smooth muscle cell proliferation compared with BAE-NEO1. (A) Serum-free conditioned media was generated from BAE-NEO1 and BAE-AP3 cells, assayed for total protein concentration, and then subjected to ion exchange chromatography to isolate the proteoglycan fraction. The total amount of glycosaminoglycan and protein determined in BAE-NEO1 (shaded bars) and BAE-AP3 (filled bars) (see Materials and Methods). (B) Western blot for perlecan of conditioned media-derived proteoglycan from BAE-NEO1 (left lane) and BAE-AP3 (right lane). (C) Inhibition of FGF-2-mediated smooth muscle cell proliferation by BAE, BAE-NEO1, and BAE-AP2 cells when grown in coculture. VSMC proliferation was analyzed in coculture with BAE cells as described (10). Confluent monolayers of BAE cells were established in Falcon cell culture inserts (0.45 μm pore size, Cyclopore Membrane, Falcon). Sparse VSMC cultures (4,000 cells/well) were established in separate six-well plates (Falcon). The media were changed to DMEM, 5% calf serum in both the BAE and VSMC cultures, and the BAE-containing culture inserts were transferred on top of the underlying VSMC. FGF-2 was administered continuously by controlled release from an alginate microsphere system within the lower (VSMC containing) chamber (42). VSMC cell number was determined after 5 days by counting trypsin suspended cells with a Coulter Counter (Model Z2). The results represent the average ± SEM stimulation of cell growth by FGF-2 [FGF-2 stimulation = (cell no. with FGF-2/cell no. without FGF-2)]. For example, the VSMC number in wells with “no cells” in the coculture insert was 1.5 × 104 and 3.3 × 104 per well in the absence and presence of FGF-2, respectively.