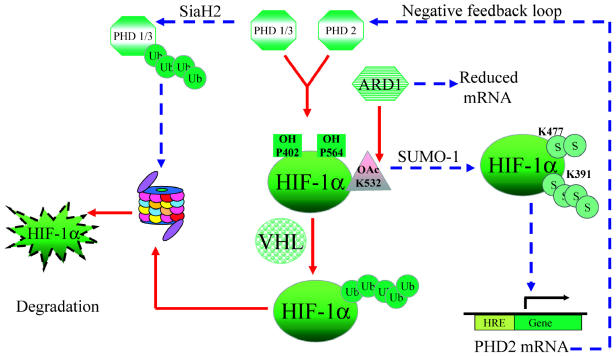
Fig. 3.
Factors affecting HIF-1α protein stability. PHD-mediated hydroxylations and ARD-mediated acetylation of specific residues within HIF-1α increase its affinity for pVHL, which leads to its ubiquitination (Ub) and degradation by the proteasomal pathway under normoxia (solid arrows). PHD1, 2, and 3 have a reduced catalytic activity in the absence of oxygen. Further, PHD1 and 3 and ARD have reduced levels in hypoxia (dashed arrows), adding another level of control. SUMO-1-mediated sumoylation in hypoxia leads to HIF-1α stabilization (S) and activation, causing transactivation of specific downstream target genes. PHD2 is induced by HIF, which indicates a negative feedback loop.