Abstract
Increased interstitial fluid pressure (IFP) in brain tumors results in rapid removal of drugs from tumor extracellular space. We studied the effects of dexamethasone and hypothermia on IFP in s.c. RG-2 rat gliomas, because they could potentially be useful as means of maintaining drug concentrations in human brain tumors. We used dexamethasone, external hypothermia, combined dexamethasone and hypothermia, and infusions of room temperature saline versus chilled saline. We measured tumor IFP and efflux half-time of 14C-sucrose from tumors. In untreated s.c. tumors, IFP was 9.1 ± 2.1 mmHg, tumor temperature was 33.7°C ± 0.7°C, and efflux half-time was 7.3 ± 0.7 min. Externally induced hypothermia decreased tumor temperature to 8.9°C ± 2.9°C, tumor IFP decreased to 3.2 ± 1.1 mmHg, and efflux half-time increased to 13.5 min. Dexamethasone decreased IFP to 2.4 ± 1.0 mmHg and increased efflux half-time to 15.4 min. Combined hypothermia and dexamethasone further increased the efflux half-time to 17.6 min. We tried to lower the tumor temperature by chilling the infusion solution, but at an infusion rate of 48 μl/min, the efflux rate was the same for room temperature saline and 15°C saline. The efflux rate was increased in both infusion groups, which suggests that efflux due to tumor IFP and that of the infusate were additive. Since lowering tumor IFP decreases efflux from brain tumors, it provides a means to increase drug residence time, which in turn increases the time-concentration exposure product of therapeutic drug available to tumor.
Keywords: brain tumor, chemotherapy, drug delivery, hypothermia, interstitial fluid pressure
Young et al. (1950) first reported increased hydrostatic pressure in tumors. More recently, Boucher et al. (1990) showed that interstitial fluid pressure (IFP)3 was increased in intracranial tumors in both patients and experimental rat tumor models. It is now recognized that the IFP of most solid tumors is increased (Heldin et al., 2004). In normal systemic tissue, net fluid movement across capillaries is determined by the hydrostatic and osmotic pressure of capillaries, as described by Starling’s equation (Jain, 1987). In systemic tissue, most of the fluid filtered into the interstitial space is reabsorbed into the venous side of the microvascular network or by the lymphatic system.
In normal brain, the blood-brain barrier (BBB) effectively nullifies the Starling equation, and solutes cross the BBB by passive diffusion. Because there is no lymphatic system in the brain, the extrachoroidal production of extracellular fluid removes excess interstitial fluid by diffusion in gray matter and bulk flow in white matter. Consequently, the IFP is close to 0 mmHg in normal brain tissue at steady state (Rosenberg et al., 1980). In brain tumors, the mechanisms responsible for increased tumor IFP are not fully understood, but increased vascular permeability, coupled with high resistance to fluid flow in the distorted interstitial space of the surrounding gray and white matter, is believed to be the main cause of increased IFP. In addition, although IFP is elevated, it is not uniform throughout a tumor (Boucher et al., 1990; Netti et al., 1995). It is elevated through the center of a tumor and drops abruptly in the tumor periphery or in the surrounding normal tissue. Based on solute distribution patterns, there is also marked heterogeneity of IFP within individual tumors (Vavra et al., 2004). The consequent pressure gradient drives interstitial fluid into the surrounding normal brain tissue, causing peritumoral edema (Baxter and Jain, 1989).
Increased IFP in brain tumors could be partially responsible for the poor delivery and distribution of both systemically administered and directly infused chemotherapeutic drugs to tumors. Increased IFP in viable tumor with increased permeability produces a bulk flow pressure gradient that moves drugs into necrotic areas or into the surrounding normal brain tissue. We previously demonstrated that, 120 min after i.v. administration of 14C-sucrose in animals with s.c. RG-2 tumors, tissue radioactivity of the isotope was found mainly in necrotic areas and in tissue surrounding the tumors (Vavra et al., 2004). In the same experiments, we found that tissue radioactivity of 14C-sucrose after convection-enhanced delivery (CED) into s.c. RG-2 tumors was also preferentially found in the necrotic areas and outside the tumor margin and that there were areas of viable tumor where the IFP was elevated that contained negligible amounts of radioactivity (Vavra et al., 2004).
We hypothesized that reducing tumor IFP would decrease the efflux rate from the tumors and thereby increase the residence time of drugs within the tumors. We used s.c. RG-2 tumors in this study: Subcutaneous and intracerebral RG-2 rat gliomas have similar microvascular structure and vascular physiology (Molnar et al., 1999; Schlageter et al., 1999), which enabled us to use s.c. tumors for ease of access in these experiments. The tumors were treated with three different protocols to lower tumor IFP: dexamethasone, hypothermia, and combined dexamethasone-hypothermia. In these groups, hypothermia was applied by external cooling. We measured temperature and interstitial pressure, and we measured the efflux rate of 14C-sucrose, which was infused in a volume of 5 μl over 1 min to minimize disturbances of the existing tumor IFP. Once the hypothermia experiments had been shown to reduce IFP, two additional experimental groups were used to evaluate the effect of a CED infusion on efflux kinetics: Tumors were infused with room temperature saline or chilled saline (containing 14C-sucrose) at a rate of 48 μl/min for 15 min. These groups were included because of the inherent difficulties associated with cooling an entire tumor in a clinical situation, as opposed to the ease of cooling the infusing solution as a means of reducing temperature.
Materials and Methods
Tumor Transplantation
All animal experimentation was reviewed and approved by the Institutional Animal Care and Use Committee of Evanston Northwestern Healthcare Research Institute. Fischer-344 rats (Harlan Industries, Indianapolis, Ind.) were anesthetized with isoflurane/nitrous oxide/oxygen anesthesia (1.5/30/70; v/v/v). Ten microliters of RG-2 glioma cells (106 cells/ml) was injected into subcutaneous tissue in the flank as described previously (Groothuis et al., 1983).
Interstitial Fluid Pressure Measurement
Tumor IFP was measured with the wick-in-needle technique described by Fadnes et al. (1977) using a 23-gauge needle. Five nylon surgical sutures (6-0 Novafil; D&G Monofil Inc., Manati, P.R.) were placed within the needle. To take pressure measurements, the needle was connected to a pressure monitor (VT-15-C; Winston Electronics Company, Millbrae, Calif.) by PE-50 polyethylene tubing filled with sterile heparinized saline (70 units/ml; American Pharmaceutical Partners, Inc., Los Angeles, Calif.). Prior to each pressure measurement, the calibration of the pressure-monitoring machine was verified. After the needle was inserted into the tumor, the IFP was read after a delay of 5 min or after the pressure stabilized.
Experimental Procedures
To study the effects of hypothermia and dexamethasone on IFP, 27 Fischer-344 rats bearing 5- to 8-mm-diameter s.c. RG-2 tumors were randomized into three experimental groups. After an i.p. injection of ketamine (50 mg/kg) and xylazine (7.5 mg/kg), tumor size was measured, and the tumor volume was calculated by using the formula V = 0.4 × ab2, where a and b are the longest and shortest diameters of the tumors, respectively (Lee et al., 1992).
In the first group (n = 9), the tumor IFP was measured before and after the tumor temperature was lowered by an application of ice over the tumors. The skin around the tumor was shaved and Vaseline applied to the skin. A plastic ring was placed around tumors to contain ice water over the tumors. The IFP before the tumor temperature was reduced was used as a baseline IFP. Tumor temperature was recorded with a temperature probe (model 43TA; Yellow Springs Instruments, Yellow Springs, Ohio) subcutaneously inserted next to the tumor capsule. After the tumor temperature was lowered and stabilized, the IFP was recorded a second time, and then 5 μl of 14C-sucrose (5 μCi) {[14C(U)]-sucrose; Movarek Biochemicals, Brea, Calif.} was injected into the tumor over 1 min by using a gastight syringe with an attached needle. Duplicate 1-μl samples of infusate were collected and counted with a liquid scintillation counter to document the amount of isotope infused into the animals. Tumor temperature and IFP were recorded at 1-min intervals to ensure that both tumor temperature and IFP were stabilized during the experiment. The rats were euthanized, and the tumors were removed at 5, 10, and 15 min (n = 3 for each time point) after completion of 14C-sucrose injection. All tumors were rapidly dissected from the skin and surrounding connective tissue, frozen in liquid tetrafluoroethane (Cytocool II Tissue Freezing Aerosol [stored at −80°C]; Stephens Scientific, Riverdale, N.J.) within 1 min of the conclusion of the experiment, weighed, and then homogenized. Radioactivity concentration in the homogenate was determined by liquid scintillation counting with appropriate quenched 14C standards (Tri-Carb Liquid Scintillation Analyzer model 2300; Packard Instrument Co, Meriden, Conn.).
In the second group (n = 9), rats were injected i.p. with dexamethasone (3 mg/kg; American Regent Laboratories, Inc., Shirley, N.Y.). Twenty-four hours later, the IFP was recorded, 5 μl of 14C-sucrose (5 μCi) was injected into the tumors over 1 min, the animals were euthanized, and the tumors were removed at 5, 10, and 15 min (n = 3 for each time point) after 14C-sucrose injection. The tumors were analyzed as described above.
In the third group (n = 9), rats were injected i.p. with dexamethasone (3 mg/kg; American Regent Laboratories, Inc.) 24 h hours prior to the experiment. The tumors were prepared for hypothermia as described above. In these experiments, IFP was not recorded. Five microliters of 14C-sucrose (5 μCi) was injected into the tumors over 1 min, the animals were euthanized, and the tumors were removed at 5, 10, and 15 min (n = 3 for each time point) after 14C-sucrose injection. The tumors were analyzed as described above.
In the groups to analyze the effects of chilling the CED infusate, saline was infused at a rate of 48 μl/min into s.c. RG-2 tumors; nine rats received an infusion of room temperature saline and nine received an infusion in which the infusion syringe and tubing were placed in ice and cooled to 15°C. Both infusates contained 10 μCi/ml of 14C-sucrose. Temperature around the tumor and tumor IFP were recorded each minute. The tumors were removed at 5, 10, and 15 min following the start of the infusion and prepared for liquid scintillation counting as described above.
Analysis
The efflux constant was determined by using the method described by Cserr et al. (1981). Normalized tissue radioactivity concentration was plotted against time and the data fit to a monoexponential expression:
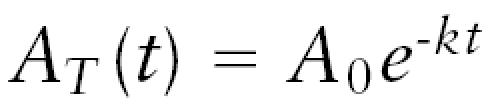 |
where AT(t) is the terminal tissue radioactivity concentration at time t, A0 is the starting tissue radioactivity concentration, and k is the first-order rate constant for total 14C-sucrose efflux from the tumors. The data were fit with nonlinear least-squares methods.
Student’s t-test was used for comparison of data from the hypothermia group, the dexamethasone group, and the control group. Paired t-tests were used to compare the serial temperature and IFP measurements during chilled infusion experiments.
Results
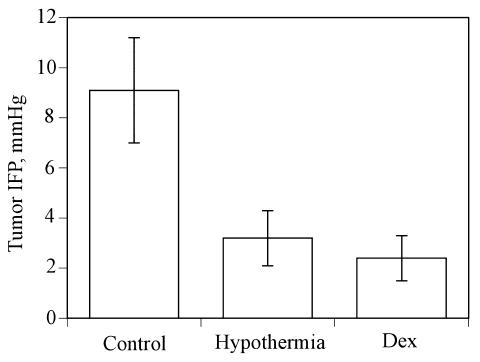
The tumor volume (mean ± SD) of s.c. RG-2 tumors was 91.1 ± 29.4 mm3 in the group with externally applied hypothermia, 99.5 ± 41.5 mm3 in the group treated with dexamethasone, and 109.4 ± 22.4 mm3 in the combined dexamethasone-hypothermia group. In the hypothermia group, the average initial tumor temperature was 33.7°C ± 0.75°C, which decreased by 8.9°C ± 2.9°C after application of ice. In this group of rats, the initial tumor IFP in the s.c. RG-2 tumors was 9.1 ± 2.1 mmHg, with a range of 6.8 to 14 mmHg. Hypothermia decreased the IFP to 3.2 ± 1.1 mmHg (range, 2.0–5.5 mmHg). Dexamethasone lowered the mean tumor IFP to 2.4 ± 0.9 mmHg (range, 1.5–4.0 mmHg). IFP was not measured in the combined dexamethasone-hypothermia group. Figure 1 illustrates the IFP values of the control, hypothermia, and dexamethasone groups; in percentage terms, hypothermia and dexamethasone reduced the tumor IFP by 64.7% and 73.6%, respectively. The values of the hypothermia and dexamethasone groups were significantly lower than those of the control group (P < 0.01, Student’s t-test).
Fig. 1.
The effect of hypothermia and dexamethasone on tumor IFP. In contrast to the IFP in untreated control RG-2 tumors (Control), tumor IFP was significantly lowered (P < 0.005) by decreasing tumor temperature (Hypothermia) and by administration of dexamethasone (Dex). Values are shown as mean ± SD.
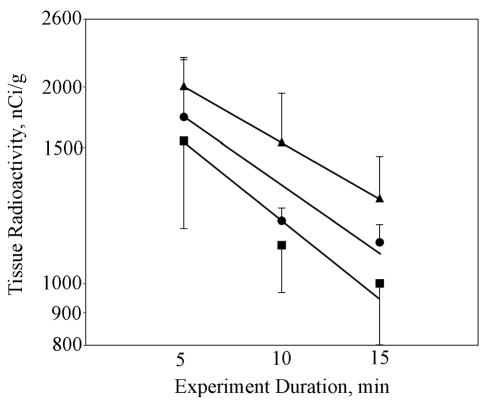
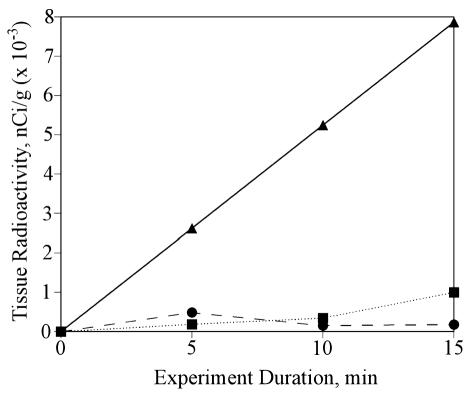
Tissue radioactivity concentration versus experiment duration is shown in Fig. 2 for the hypothermia, dexamethasone, and combined hypothermia-dexamethasone groups. The hypothermia group had an efflux rate constant of 0.0515 min−1 (r2 = 0.9337), which corresponds to an efflux half-time of 13.5 min. The dexamethasone group had an efflux rate constant of 0.0451 min−1 (r2 = 0.8741), which corresponds to an efflux half-time of 15.4 min. The combined hypothermia-dexamethasone group had an efflux rate constant of 0.0404 min−1 (r2 = 0.99), which corresponds to an efflux half-time of 17.2 min. Examination of the data from the hypothermia and dexamethasone groups shows a change in the slope from 5 to 10 min and 10 to 15 min, suggesting that the effects might have not been constant during the experimental period.
Fig. 2.
Tissue radioactivity values versus time in three treatment groups. Shown are the data (±SD) and linear least-squares fits for the hypothermia group (r2 = 0.9337) (▪), dexamethasone group (r2 = 0.8741) (•), and combined hypothermia-dexamethasone group (r2 = 1.0) (▴). Efflux half-times were calculated from an exponential fit to the data.
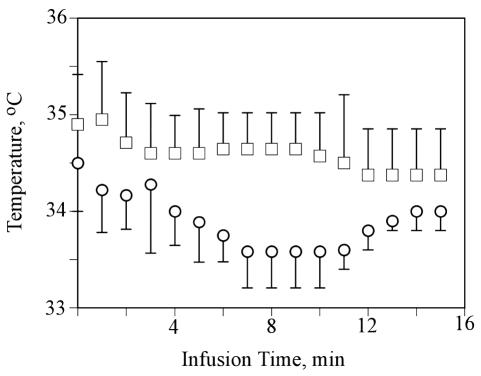
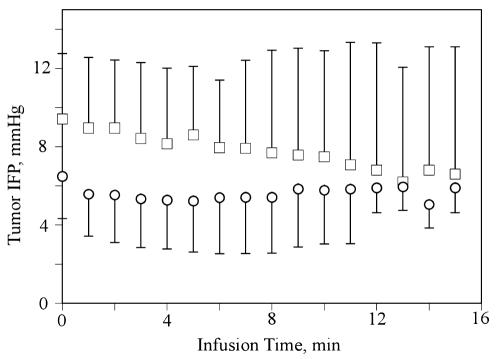
In the other experiments, which examined the effect of chilling the infusate, the results of the temperature measurements are shown in Fig. 3 and IFP measurements in Fig. 4. Despite cooling the infusate to 15°C, the temperature recorded by the probe, which was located next to the tumor, never showed more than a 1.5°C difference between the normal and chilled saline groups (Fig. 3) (P > 0.01, paired t-test). Likewise, although there was an initial difference in IFP between the two groups (Fig. 4), neither group showed any significant change in the tumor IFP over the 15-min experiment (P > 0.01, paired t-test). Figure 5 shows a plot of tissue radioactivity versus experiment duration for the two groups of rats. Also shown in Fig. 5 is an expected tissue radioactivity concentration, given the radioactivity concentration that was measured in the infusate and the experiment duration. Surprisingly, almost no isotope was found in the tumors at any time point in either group; that is, the isotope left the tumor at such a high rate that the data could not be used to calculate an efflux rate constant.
Fig. 3.
Temperature versus time in RG-2 tumors receiving CED infusions. The temperatures (°C ± SD) are shown for the group receiving a 48-μl/min infusion of saline containing 14C-sucrose at room temperature (□) or chilled to 15°C (○). The differences were not statistically significant.
Fig. 4.
Tumor interstitial pressure versus time in RG-2 tumors receiving CED infusions. Tumor IFP (±SD) was recorded every minute during a 15-min infusion at 48 μl/min of saline containing 14C-sucrose at room temperature (□) and chilled to 15°C (○). The differences were not statistically significant.
Fig. 5.
Tissue radioactivity values versus time in treatment groups of infusion of room temperature saline versus saline chilled to 15°C. There is almost no radioactivity remaining in the tumors of the room temperature saline group (▪) or the chilled saline group (•). The triangles (▴) show the expected amount of radioactivity that would be in the tumors if none of the 14C-sucrose had left.
Discussion
We previously reported that the efflux half-time of 14C-sucrose from s.c. RG-2 tumors was 7.3 ± 0.7 min (Vavra et al., 2004). In the present study, we demonstrated that the efflux half-time of 14C-sucrose from s.c. RG-2 tumors could be increased by lowering tumor IFP. Externally applied hypothermia, which decreased temperature by a modest amount (8.9°C ± 2.9°C), and dexamethasone both decreased the efflux rate (Fig. 2), and furthermore, the effect was additive (Fig. 2). Since the mean residence time, which is the average time that a drug molecule remains in the tumor, is equal to the reciprocal of the elimination (efflux) rate constant, each of these manipulations would effectively increase the residence time of a drug in tumor. For example, a mean residence time that for untreated RG-2 tumors was 10.56 min would be increased to 19.42 min after hypothermia, 22.17 min after dexamethasone, and 24.75 min for the combined hypothermia-dexamethasone group.
In contrast, we were surprised by the lack of effect of a high-flow (48-μl/min) infusion on the efflux of radio-label from the s.c. RG-2 tumors. We used 48 μl/min because it was a rate at which we could overcome IFP in RG-2 tumors and achieve a clear therapeutic effect (Ali et al., 2006), but in addition, we reasoned that delivering a large volume of chilled solute would give the best chance of detecting an effect from the hypothermic infusion. The results from the room temperature saline infusions indicated that the isotope was leaving too rapidly to measure with the time points we selected (Fig. 5). This meant that the efflux rate normally present in RG-2 gliomas was even further increased by the infusion; less than 10% of the infused isotope remained in the tumors after 5 min (Fig. 5). Chilling the infusate neither reduced the temperature in the probe located next to the tumor (Fig. 3) nor did it affect IFP (Fig. 4). In contrast to the first set of experiments in which ice-cold water was placed on top of the tumor, in these, saline chilled to 15°C was infused into the tumor. Although 48 μl/min is a high infusion rate, it nonetheless represents a total volume of only 0.24 ml over 5 min, which represents less than one fourth of the volume of these s.c. tumors. In effect, the temperature was buffered by the tumor and surrounding tissue, and the infusion increased efflux from the tumor. Although we do not believe this approach is likely to be clinically feasible, it does deserve further exploration because of the ease with which it could be used.
The mechanisms that determine tumor IFP are not fully understood, but an understanding of the fundamental concepts is important to develop ideas about how to manipulate it. One source of the pressure gradient almost certainly involves increased vascular permeability (Boucher and Jain, 1992; Netti et al., 1995). RG-2 capillaries have increased populations of both fenestrations and large interendothelial gaps that will allow transcapillary movement of both small and large solutes (Schlageter et al., 1999). This represents a major change from the condition in normal brain. The Starling equation, which expresses bulk flow in terms of hydrostatic and osmotic gradients across the capillary wall, shows the nature of this change:
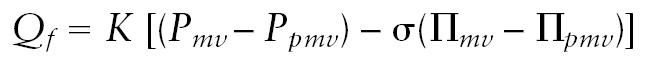 |
In this equation, Qf is the filtration rate, K is the capillary filtration coefficient, σ is the reflection coefficient, and P and Π are hydrostatic pressure and osmotic pressure, respectively, where mv represents the intravascular pressure and pmv represents the perimicrovascular pressure. In normal brain, K and σ approach values of 0, and there is essentially no bulk flow (i.e., Qf ⇒ 0); consequently, solutes can cross the BBB only by simple diffusion. The Starling equation illustrates the importance of local pressures in solute movement once the BBB has been structurally altered.
Once water and solutes have crossed into the extracellular space, the rate of bulk flow between two regions is determined by the pressure gradient and the resistance offered by the extracellular space, as described by D’Arcy’s law:
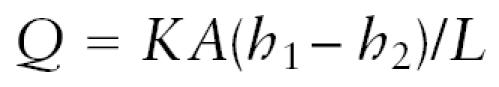 |
where Q is volume flow (volume per unit time), K is a coefficient that relates the resistance of the medium to the solvent and solutes, A is the cross-sectional area, h1 and h2 are the pressures at the source and terminus, respectively, and L is the distance between the two.
These two equations can help identify critical control points at which bulk flow in a tumor can be influenced, keeping in mind that reducing bulk flow increases the residence time and exposure of tumor cells to a therapeutic compound. For instance, reducing transcapillary volume flow Qf, increasing tissue resistance (K), and reducing the tissue interstitial fluid pressure gradient (h1 – h2) are each mechanisms for reducing bulk flow. We need a better understanding about the fluid mechanics involved (Bear, 1988) in the tumors as well as the biological control points. Several studies have shown that many agents can lower tumor IFP and improve tumor uptake of chemotherapeutic drugs through various mechanisms of drug action (Curnis et al., 2002; Emerich et al., 1998; Pietras et al., 2002; Salnikov et al., 2003; Willett et al., 2004). It should also be mentioned that we currently have no noninvasive methods for measuring K or IFP in humans, but as our understanding of drug delivery to brain tumor cells improves, it may become important to measure these values directly or indirectly.
Dexamethasone probably has complex mechanisms of action. It has been reported to reduce tumor IFP (Heldin et al., 2004; Kristjansen et al., 1993). Although dexamethasone has been reported to decrease capillary permeability in brain tumors (Jarden et al., 1985, 1989), we were not been able to demonstrate such an effect in RG-2 gliomas (Molnar et al., 1995; Nakagawa et al., 1987, 1988). However, dexamethasone is a powerful corticosteroid that probably mediates an effect on IFP by many other pathways (Guerin et al., 1992; Heiss et al., 1996; Ikeda et al., 1993; Kiely et al., 1994; Wolff et al., 1993). The effect of dexamethasone on IFP in brain tumors needs to be studied more, and studies should include physiological experiments to better understand the time course and magnitude of dose-effect relationships and biological experiments to better determine the mechanisms of its action. Since dexamethasone is widely used in treating edema in brain tumor patients, a better understanding of its actions could yield information about maximizing its effects on efflux and improving the availability of therapeutic drugs to brain tumor cells.
The effect of temperature on IFP has also not been fully investigated, but is perhaps even more complex than that of dexamethasone. Paradoxically, hyperthermia can also lower tumor IFP, which was assumed to be due to tumor microcirculation damage from the hyperthermia (Leunig et al., 1992). However, the effect of hypothermia is potentially much more manageable. Cooling reduces blood flow and metabolism in normal brain (Strauch et al., 2005). The reduced IFP achieved by a decrease in tumor temperature could be the result of decreased production of water, either from capillary permeability or due to reduced water production from cellular metabolism. In either case, cooling offers a transient, reversible method of reducing IFP that could be used during direct drug infusions into tumors or surrounding brain. A recent cooperative study in subarachnoid hemorrhage did not show a benefit of mild cooling during surgery for intracranial aneurysm, but did establish the safety and efficacy of the approach (Todd et al., 2005), and it has proven safe in neonates (Shankkaran et al., 2005). If drug delivery turns out to be a major obstacle in brain tumor chemotherapy, the ultimate method to reduce IFP by hypothermia would be to induce circulatory arrest, which would probably reduce tumor IFP close to 0. Hypothermic circulatory arrest is now used in aortic surgery for up to 30 min without neurologic morbidity (Kunihara et al., 2005). Before this can be used in brain tumor patients, the delivery advantages in terms of improved drug distribution and lengthened residence time need to be explored more fully.
Vavra et al. (2004) demonstrated the effect of increased tumor IFP on the distribution of systemically administered or CED-administered 14C-sucrose inside tumors and the increased rate of drug efflux from the tumors. Negligible amounts of isotope were found in areas of viable tumor, while the isotope preferentially distributed in areas of necrosis and at the tumor margin where the tumor IFP gradient decreased. When drugs are administered by CED, areas of viable tumor are likely to receive reduced exposure to therapeutic agents because they will have high IFP values, effectively creating a pressure barrier that keeps the infusate from entering these tumor regions. Lowering the tumor IFP could reduce the pressure gradient and increase drug residence time inside the tumor. This would result in an increase in the time that the tumor cells are exposed to therapeutic drugs and improve chances of eradicating the tumor. In addition to the issue of effective chemotherapeutic drugs, the delivery of those drugs in sufficient amounts to kill tumor cells is a crucial factor in the successful treatment of tumor patients. Understanding tumor physiology would help to design a treatment regimen suitable for an individual patient. The type of drug, administration route, manipulation of tumor physiology (e.g., lowering the tumor IFP as demonstrated in this study), dosage, and interval could be planned to maximize drug delivery to tumors and offer patients the best treatment to cure their disease.
Footnotes
This work was supported by NIH grant R01-NS12745 and by the Richard M. Lilienfield Memorial Fund. Y. Navalitloha was supported by the Ida and Irving Abraham Family Foundation, and D.R. Groothuis was supported by the Stanley C. Golder Chair of Neuroscience Research.
Abbreviations used are as follows: BBB blood-brain barrier; CED convection-enhanced delivery; IFP interstitial fluid pressure.
References
- Ali MJ, Navalitloha Y, Vavra M, Kang E.W.-Y, Itskovich AC, Molnar P, Levy RM, Groothuis DR. Isolation of drug delivery from drug effect: Problems of optimizing drug delivery parameters. Neuro-Oncology. 2006;8:109–118. doi: 10.1215/15228517-2005-007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. I Role of interstitial pressure and convection. Microvasc Res. 1989;37:77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Bear, J. (1988) Dynamics of Fluids in Porous Media New York: Dover Publications, Inc.
- Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue isolated and subcutaneous tumors: Implications for therapy. Cancer Res. 1990;50:4478–4484. [PubMed] [Google Scholar]
- Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: Implications for vascular collapse. Cancer Res. 1992;52:5110–5114. [PubMed] [Google Scholar]
- Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radio-labeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240:F319–F328. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest. 2002;110:475–482. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Snodgrass P, Pink M, Bloom F, Bartus RT. Central analgesic actions of loperamide following the transient permeation of the blood brain barrier with Cereport (RMP-7) Brain Res. 1998;801:259–266. doi: 10.1016/s0006-8993(98)00571-x. [DOI] [PubMed] [Google Scholar]
- Fadnes HO, Reed RK, Aukland K. Interstitial fluid pressure in rats measured with a modified wick technique. Microvasc Res. 1977;14:27–36. doi: 10.1016/0026-2862(77)90138-8. [DOI] [PubMed] [Google Scholar]
- Groothuis DR, Pasternak JF, Fischer JM, Blasberg RG, Bigner DD, Vick NA. Regional measurements of blood flow in transplanted RG-2 rat gliomas. Cancer Res. 1983;43:3362–3367. [PubMed] [Google Scholar]
- Guerin C, Wolff JE, Laterra J, Drewes LR, Brem H, Goldstein GW. Vascular differentiation and glucose transporter expression in rat gliomas: Effects of steroids. Ann Neurol. 1992;31:481–487. doi: 10.1002/ana.410310504. [DOI] [PubMed] [Google Scholar]
- Heiss JD, Papavassiliou E, Merrill MJ, Nieman L, Knightly JJ, Walbridge S, Edwards NA, Oldfield EH. Mechanism of dexamethasone suppression of brain tumor–associated vascular permeability in rats: Involvement of the glucocorticoid receptor and vascular permeability factor. J Clin Invest. 1996;98:1400–1408. doi: 10.1172/JCI118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C.-H, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Carson BS, Lauer JA, Long DM. Therapeutic effects of local delivery of dexamethasone on experimental brain tumors and peritumoral brain edema. J Neurosurg. 1993;79:716–721. doi: 10.3171/jns.1993.79.5.0716. [DOI] [PubMed] [Google Scholar]
- Jain RK. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- Jarden JO, Dhawan V, Poltorak A, Posner JB, Rottenberg DA. Positron emission tomographic measurement of blood-to-brain and blood-to-tumor transport of 82Rb: The effect of dexamethasone and whole-brain radiation therapy. Ann Neurol. 1985;18:636–646. doi: 10.1002/ana.410180603. [DOI] [PubMed] [Google Scholar]
- Jarden JO, Dhawan V, Moeller JR, Strother SC, Rottenberg DA. The time course of steroid action on blood-to-brain and blood-to-tumor transport of 82Rb: A positron emission tomographic study. Ann Neurol. 1989;25:239–245. doi: 10.1002/ana.410250306. [DOI] [PubMed] [Google Scholar]
- Kiely J, Hadcock JR, Bahouth SW, Malbon CC. Glucocorticoids down-regulate beta-1 adrenergic-receptor expression by suppressing transcription of the receptor gene. Biochem J. 1994;302:397–403. doi: 10.1042/bj3020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansen PE, Boucher Y, Jain RK. Dexamethasone reduces the interstitial fluid pressure in a human colon adenocarcinoma xeno-graft. Cancer Res. 1993;53:4764–4766. [PubMed] [Google Scholar]
- Kunihara T, Grun T, Aicher D, Langer F, Adam O, Wendler O, Saijo Y, Schafers H. Hypothermic circulatory arrest is not a risk factor for neurologic morbidity in aortic surgery: A propensity score analysis. J Thorac Cardiovasc Surg. 2005;130:712–718. doi: 10.1016/j.jtcvs.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Lee I, Boucher Y, Jain RK. Nicotinamide can lower tumor interstitial fluid pressure: Mechanistic and therapeutic implications. Cancer Res. 1992;52:3237–3240. [PubMed] [Google Scholar]
- Leunig M, Goetz AE, Dellian M, Zetterer G, Gamarra F, Jain RK, Messmer K. Interstitial fluid pressure in solid tumors following hyperthermia: Possible correlation with therapeutic response. Cancer Res. 1992;52:487–490. [PubMed] [Google Scholar]
- Molnar P, Fekete I, Schlageter KE, Lapin GD, Groothuis DR. Absence of host-site influence on angiogenesis, blood flow, and permeability in transplanted RG-2 gliomas. Drug Metab Dispos. 1999;27:1085–1091. [PubMed] [Google Scholar]
- Molnar P, Lapin GD, Groothuis DR. The effects of dexamethasone on experimental brain tumors: I. Transcapillary transport and blood blow in RG-2 rat gliomas. J Neurooncol. 1995;25:19–28. doi: 10.1007/BF01054719. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Groothuis DR, Owens ES, Fenstermacher JD, Patlak CS, Blasberg RG. Dexamethasone effects on [125I]albumin distribution in experimental RG-2 gliomas and adjacent brain. J Cereb Blood Flow Metab. 1987;7:687–701. doi: 10.1038/jcbfm.1987.123. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Groothuis DR, Owens ES, Patlak CS, Blasberg RG. Dexamethasone effects on vascular volume and tissue hematocrit in experimental RG-2 gliomas and adjacent brain. J Neuro-oncol. 1988;6:157–168. doi: 10.1007/BF02327392. [DOI] [PubMed] [Google Scholar]
- Netti PA, Baxter LT, Boucher Y, Skalak R, Jain RK. Time-dependent behavior of interstitial fluid pressure in solid tumors: Implications for drug delivery. Cancer Res. 1995;55:5451–5458. [PubMed] [Google Scholar]
- Pietras K, Rubin K, Sjoblom T, Buchdunger E, Sjoquist M, Heldin CH, Ostman A. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–5484. [PubMed] [Google Scholar]
- Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol. 1980;238:F42–F49. doi: 10.1152/ajprenal.1980.238.1.F42. [DOI] [PubMed] [Google Scholar]
- Salnikov AV, Iversen VV, Koisti M, Sundberg C, Johansson L, Stuhr LB, Sjoquist M, Ahlstrom H, Reed RK, Rubin K. Lowering of tumor interstitial fluid pressure specifically augments efficacy of chemotherapy. FASEB J. 2003;17:1756–1758. doi: 10.1096/fj.02-1201fje. [DOI] [PubMed] [Google Scholar]
- Schlageter KE, Molnar P, Lapin GD, Groothuis DR. Microvessel organization and structure in experimental brain tumors: Microvessel populations with distinctive structural and functional properties. Microvasc Res. 1999;58:312–328. doi: 10.1006/mvre.1999.2188. [DOI] [PubMed] [Google Scholar]
- Shankkaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Strauch JT, Spielvogel D, Lauten A, Zhang N, Rinke S, Weisz D, Bodian CA, Griepp RB. Optimal temperature for selective cerebral perfusion. J Thorac Cardiovasc Surg. 2005;130:74–82. doi: 10.1016/j.jtcvs.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Todd MM, Hindman BJ, Clarke WR, Torner JC. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135–145. doi: 10.1056/NEJMoa040975. [DOI] [PubMed] [Google Scholar]
- Vavra M, Ali MJ, Kang E.W.-K, Allen CA, Groothuis DR. Comparative pharmacokinetics of 14C-sucrose delivery to RG-2 rat gliomas by intravenous and convection-enhanced delivery. Neuro-Oncology. 2004;6:104–112. doi: 10.1215/S1152851703000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer (erratum in Nat. Med. [2004] 10, 649). . Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JE, Guerin C, Laterra J, Bressler J, Indurti RR, Brem H, Goldstein GW. Dexamethasone reduces vascular density and plasminogen activator activity in 9L rat brain tumors. Brain Res. 1993;604:79–85. doi: 10.1016/0006-8993(93)90354-p. [DOI] [PubMed] [Google Scholar]
- Young JS, Lumsden CE, Stalker AL. The significance of the tissue pressure of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J Pathol Bacteriol. 1950;62:313–333. doi: 10.1002/path.1700620303. [DOI] [PubMed] [Google Scholar]