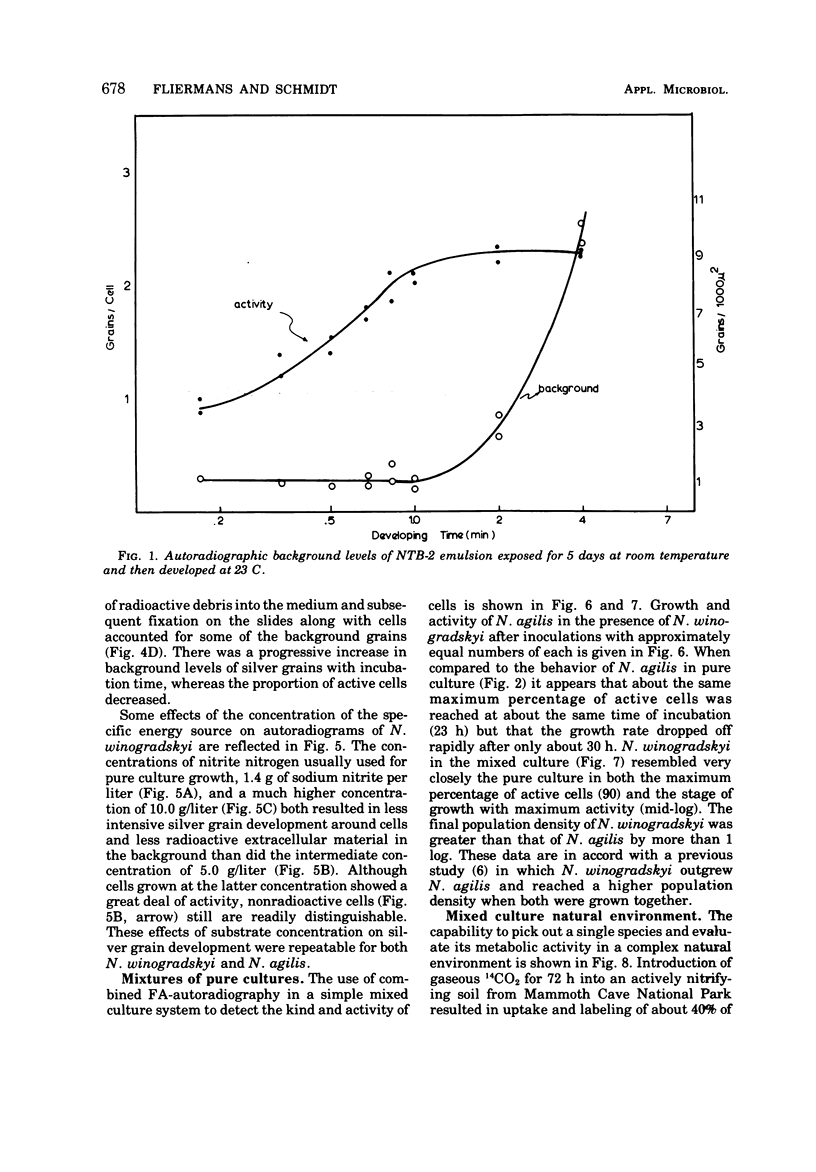
Abstract
Specific detection of a particular bacterium by immunofluorescence was combined with estimation of its metabolic activity by autoradiography. The nitrifying bacteria Nitrobacter agilis and N. winogradskyi were used as a model system. Nitrobacter were incubated with NaH14CO3 and 14CO2 prior to study. The same preparations made for autoradiograms were stained with fluorescent antibodies specific for the Nitrobacter species. Examination by epifluorescence and transmitted dark-field microscopy revealed Nitrobacter cells with and without associated silver grains. Direct detection and simultaneous evaluation of metabolic activity of Nitrobacter was demonstrated in pure cultures, in a simple mixed culture, and in a natural soil.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylen C. W. Survival of Arthrobacter crystallopoietes during prolonged periods of extreme desiccation. J Bacteriol. 1973 Jan;113(1):33–37. doi: 10.1128/jb.113.1.33-37.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALK G. J., KING R. C. RADIOAUTOGRAPHIC EFFICIENCY FOR TRITIUM AS A FUNCTION OF SECTION THICKNESS. Radiat Res. 1963 Nov;20:466–470. [PubMed] [Google Scholar]
- Fliermans C. B., Bohlool B. B., Schmidt E. L. Autecological study of the chemoautotroph Nitrobacter by immunofluorescence. Appl Microbiol. 1974 Jan;27(1):124–129. doi: 10.1128/am.27.1.124-129.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliermans C. B., Brock T. D. Ecology of sulfur-oxidizing bacteria in hot acid soils. J Bacteriol. 1972 Aug;111(2):343–350. doi: 10.1128/jb.111.2.343-350.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudelout H., Lambert R., Fripiat J. L. Molar growth yield of Nitrobacter winogradskyi during exponential growth. Arch Microbiol. 1974 Jul 4;98(2):127–131. doi: 10.1007/BF00425275. [DOI] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Fliermans C. B., Brock T. D. Technique for measuring 14 CO 2 uptake by soil microorganisms in situ. Appl Microbiol. 1972 Mar;23(3):595–600. doi: 10.1128/am.23.3.595-600.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



