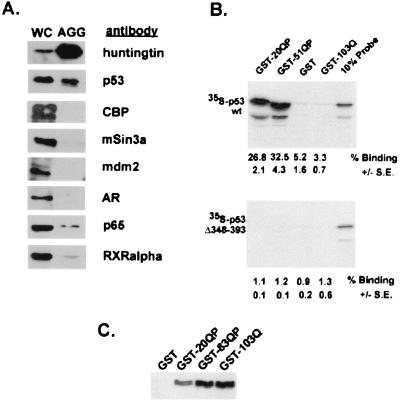
Figure 1.
p53 colocalizes with expanded httex1p in inclusions in mammalian cell culture and interacts with httex1p in vitro and in cell culture. (A) Western blot of solubilized proteins purified from aggregates generated in HEK293 cells expressing 103Q-GFP. Results were identical to aggregates purified from 103QP-GFP (data not shown). WC denotes the whole-cell fraction from transfected cells and AGG the aggregate preparation. Equivalent ratios of WC:AGG were run on 8% SDS gels and Western blotted. Antisera were used to examine relative levels of 103Q-GFP htt, p53, CBP, mSin3a, mdm2, AR, NF-κB p65, and RXRα in the aggregate preparation. No immunoreactive htt protein was present in samples corresponding to the aggregate fraction isolated from 293 cells expressing 25Q-GFP (data not shown). (B) Binding of [35S]methionine-labeled p53(1–393) and p53(1–347) to GST-htt fusion proteins. Equivalent amounts of [35S]methionine-labeled p53(1–393) and p53(1–347) were mixed with GST-20QP, GST-51QP, GST, and GST-103Q attached to glutathione-agarose beads, incubated, and washed. The labeled proteins bound to the beads were analyzed by SDS-PAGE. Each experiment was done in triplicate. Percent binding ± SE was calculated by phosophorimager analysis and is listed below each lane. Ten percent of the input of the labeled proteins was also analyzed as shown. Slight alteration in the pattern of p53 migration in the GST-51QP lane as compared with GST-20QP lane is because of large amounts GST-51QP comigrating with labeled p53. (C) Coprecipitation of p53 with httex1p in HEK293 cells. HEK293 cells were transiently transfected with plasmids encoding p53 and either GST, GST-20QP, GST-83QP, or GST-103Q. Cells were broken and lysates were incubated with glutathione-agarose beads. After extensive washing, beads were loaded directly on an 8% SDS gel. Western blot analysis using monoclonal anti-p53 antibody is shown.