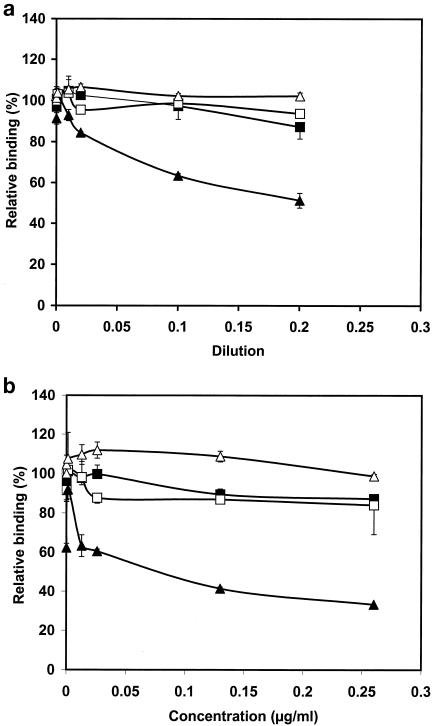
FIG. 4.
Characterization of the specificity of the affinity-purified serotype d-specific antigen. ELISA plates were coated with formalin-killed whole cells of A. actinomycetemcomitans strains representing serotypes a to e as described in Materials and Methods. The isolated fractions, the affinity-purified serotype d fraction and the control fraction, competed with the immobilized whole-cell antigen for binding to the corresponding antiserum. The binding of the antiserum was detected with peroxidase-conjugated anti-rabbit IgG. (a) Microtiter plates were coated with serotype d whole cells, and the affinity-purified serotype d-specific antigen (▴) and the control fraction (▵) competed for binding to the rabbit antiserum raised against serotype d. Microtiter plates were coated with serotype c whole cells, and affinity-purified serotype d-specific antigen (▪) and the control fraction (□) competed for the binding to the rabbit antiserum raised against serotype c. (b) As measured with the malondealdehyde-thiobarbituric acid reaction, the affinity-purified serotype d-specific antigen contained 0.13 μg of LPS per ml. Displacement ELISA was repeated with known LPS concentrations, and the affinity-purified serotype d-specific fraction and LPS isolated from E. coli (0.13 μg/ml) were used as competitors. ELISA plates were coated with A. actinomycetemcomitans serotype d whole cells, and affinity-purified serotype d-specific antigen (▴) and LPS isolated from E. coli (▵) competed for the binding to the rabbit antiserum raised against serotype d. The microtiter plates were coated with serotype c whole cells, and affinity-purified serotype d-specific antigen (▪) and LPS isolated from E. coli (□) competed for the binding to the rabbit antiserum raised against serotype c. Error bars indicate standard deviations.