Abstract
Aims
To conduct a randomized placebo controlled double-blind crossover trial in order to evaluate a loratadine-pseudoephedrine combination (L+PS) in children with seasonal allergic rhinitis.
Methods
Forty children (15 males; 25 females), aged 3–15 years, were included in this study. They were randomized to receive L+PS (0.2 mg kg−1 body weight–2.4 mg kg−1 body weight respectively) or placebo (PLA) for 14 days. After 7 days of washout, patients were shifted to the other treatment for a further 14 days. Nasal symptoms (sneezing/itching, congestion, nasal dripping) and signs (turbinal swelling, retronasal drainage), rated on a scale ranging from: 1. absent to 5. very intense, and their sum or mean total symptom score (MTSS) were used as efficacy measurement.
Results
Significant relief was observed; post-treatment MTSS difference and its percent change were respectively; L+PS = −4.29; 95% CI: −3.64 and −4.94 (27.8%), and PLA = −1.63; 95% CI: −0.95 and −2.31 (10.7%) (P < 0.001 baseline vs endpoint and between treatments). Furthermore, L+PS and PLA significantly modified symptoms, but only L+PS significantly modified signs. No clinical changes were observed during the trial; only one patient showed slight transient insomnia when receiving L+PS.
Conclusions
It is concluded that L+PS is useful and well tolerated in children with seasonal allergic rhinitis. However, elements such as placebo effect must be taken into account for planning future trials.
Keywords: loratadine-pseudoephedrine, placebo, crossover-trial, children
Introduction
Allergic rhinitis is a common disease affecting both children and adults with two variants, seasonal and perennial [1]. Seasonal rhinitis appears to be increasing in frequency and represents a common problem in paediatric practice [2]. Its main symptoms are sneezing, itching, congestion, persistent nasal dripping and turbinal swelling with nasal obstruction. Allergic rhinitis is a typical IgE mediated illness [3] although other mechanisms may contribute: Allergen binding to IgE, triggers an antigent-antibody reaction in nasal mucosa that releases inflammatory mediators such as histamine. The response is prolonged by on anterograde reflex involving substance P (neurogenic inflammation) [4]. Sneeze and itching are probably the result of histamine action whereas obstruction and dripping are induced by other mediators (i.e. prostaglandin D2 and leukotriene C4) [5].
Many factors including environmental influences [1, 6, 7] contribute to this disease. Consequently, there are several therapeutic alternatives [1, 3, 5]. The symptomatic approaches antagonize the inflammatory mediator response at the receptor or intracellular level. Effective relief of symptoms can be achieved by the combination of a peripheral antihistamine and α-adrenergic amine [3]. This kind of combination (i. e., loratadine-pseudoephedrine) has a rational basis because H1-receptor blockade potentiates vasoconstriction of nasal vessels and vice versa.
Loratadine4-(8-chloro-5,6-dihydro-11H-benzo(5,6)- cyclohepta(1,2-b)pyridin-11-ylidene)-1-piperidinecarboxylic acid, ethyl ester is a H1 piperidine antihistamine devoid of central and antimuscarinic effects with a long elimination half-life and an active metabolite [8]. Pseudoephedrine (S-(R*,R*))-α-(1-(methylamino)ethyl)-benzenemethanol, is a classical orally active α-adrenoceptor mixed agonist that causes noradrenaline release [9].
Previous data have shown that the loratadine-pseudoephedrine combination (L+PS) is more effective than its components alone [10]. A loratadine-pseudoephedrine combination is already marketed in several countries as a medicament at fixed doses.
To date, the evaluation of L+PS in children with allergic rhinitis [10–12] is limited. The purpose of the present study was to evaluate the efficacy and safety of L+PS (Alerpriv D®, Química Montpellier S.A., Argentina) in children attending out-patients with seasonal allergic rhinitis. Since many of the clinical trials are subjective and may be influenced by psychological factors [13], a comparison between L+PS and placebo was selected to determine the true antiallergic effects of drug treatment.
Methods
Patients
Forty children (15 males; 25 females), aged 3–15 years with seasonal allergic rhinitis from La Plata city area, were included in this study. Diagnosis was made on the basis of previous history, physical examination and rhinoscopy. Prick test was used to identify the causative allergens. Exclusion criteria were; drug hypersensitivity, other causes of rhinitis (common cold, influenza), other allergic disorders (except for bronchial asthma if patient had no more than one attack in a year), neoplasms, systemic diseases, severe hepatic or renal failure and concomitant use of other antihistamines or glucocorticoids.
Study design
The protocol involved a randomized placebo controlled double-blind crossover design. Prior to including children, parents were provided with an information sheet outlining the purpose and design of the trial. Both parents and patients, when appropriate, gave their written informed consent. The protocol was approved by the Institutional Review Board of Sor María Ludovica Children Hospital, La Plata, Argentina. According to the study design, children were randomly assigned to one of two treatment groups: A (8 males; 12 females) received L+PS and B (8 males; 12 females) received placebo (PLA) during the first 14 days. After 7 days of washout, patients were shifted to the other treatment, so children in group A received PLA and those in group B received L+PS for another 14 days. Both treatments were supplied as 60 ml syrup (each 100 ml of L+PS contained loratadine, 100 mg and pseudoephedrine, 1200 mg). The daily dose of loratadine (0.2 mg kg−1 body weight) was used to provide individual volume of syrup. The syrup was administered orally twice a day (approximately at 08.00 h and 20.00 h). Patients were assessed on four occassions: At the beginning, at end of the first 14 days, after the washout period and at the end of the trial. Severe adverse events, protocol violation and withdrawal of consent were counted as patient drop outs.
Measurements
During the trial the following parameters were assessed: vital signs; nasal symptoms (using the scale: 1. absent; 2. mild; 3. moderate; 4. severe; 5. very intense); nocturnal rest (using the scale: 1. excellent; 2. awake once or twice at night; 3. awake more than twice at night; 4. awake all the night); adverse events and routine laboratory tests (blood count, glucose and creatinine serum levels). Symptoms (sneezing/itching, congestion and nasal dripping) were obtained by direct questionnaire, while signs (turbinal swelling and retronasal drainage) were obtained by direct observation. Nasal symptoms/signs scales and the change in the mean of their sum (mean total symptom score or MTSS) from baseline to endpoint were used as efficacy indicators according to Dockhorn et al. [14]. Vital signs, adverse events and laboratory tests were used as indicators of tolerability. Serum IgE was measured (RAST) at the outset, but was not used as inclusion criterion because 40% of atopic subjects may have normal IgE values [15].
Statistical procedures
Parametric data were expressed as mean±standard deviation, except for age which was expressed as mean-range. Distribution data were indicated in percentages. A Student's t-test for independent samples was used to assess gender differences. Non parametric data were expressed as median—95% confidence interval (95% CI), except for MTSS which was expressed as a mean—95% CI [14]. These data were analysed by the Wilcoxon matched paired test or Friedman's ANOVA. To exclude carryover effect the Mann-Whitney U test was done on group A vs group B MTSS (L+PS plus PLA). All statistical procedures were performed using Stastistica 4.3 for Windows. All tests of significance were performed at P = 0.05 level.
Results
Of the forty children, two (1 male; 1 female of group B) were excluded for non-compliance with the study protocol. The other thirty-eight patients completed the trial. Assessment of MTSS by groups (group A = 5.90; 95% CI: 4.65 and 7.15, vs group B = 5.94; 95% CI: 4,23 and 7.66; NS) excluded carryover, thus patients were analysed as a single cohort. Table 1 shows the baseline characteristics. Although there was an excess of girls, no significant differences in other variables were observed between genders. Prick test results revealed that more than half of the children were positive for two or more home allergens (dust mite alone 29%; fungi alone 13%; both in combination with pet epithelium 58%). IgE was abnormally increased (>400 ng ml−1) in 31 children.
Table 1.
Demographic characteristics of children that completed trial.

Baseline MTSS were L+PS = 15.45; 95% CI: 14.73 and 16.16, and PLA = 15.29; 95% CI: 14.42 and 16.16 (NS). Posttreatment MTSS difference and its percent change (baseline minus post-treatment MTSS divided by baseline MTSS and multiplied by 100) were respectively; L+PS = −4.29; 95% CI: −3.64 and −4.94 (27.8%), and PLA = −1.63; 95% CI: −0.95 and −2.31 (10.7%) (P < 0.001 baseline vs endpoint and between treatments, Figure 1).
Figure 1.
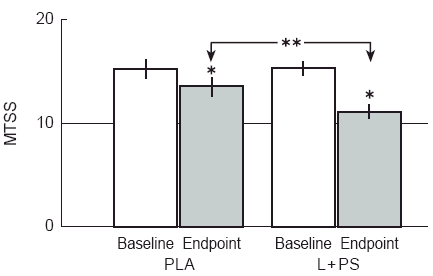
Mean total symptom score (MTSS) as a measure of global response and its variation by treatments. MTSS was obtained summing all nasal symptoms and signs (Data as mean −95% CI; n = 38). After treatment, there was a significant fall in MTSS. *, P < 0.001 baseline vs endpoint (Wilcoxon matched paired test); **, P < 0.001 between treatments (Wilcoxon matched paired test of difference baseline-endpoint).
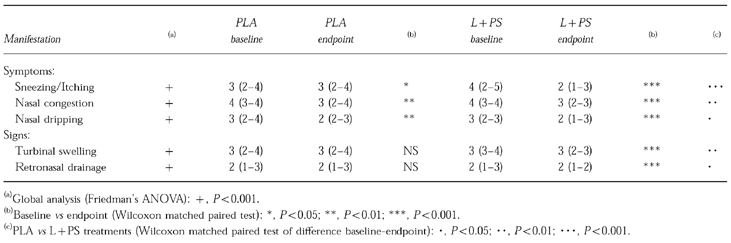
Table 2 shows the two type of nasal manifestations and their change to endpoint. Improvement of symptoms was significant with PLA but more marked with L+PS, while only signs improved significantly with L+PS.
Table 2.
Variations of nasal manifestations (n = 38). Data as median −95% CI.
One patient reported slight transient insomnia when receiving L+PS. No changes were observed in vital signs or laboratory tests along the trial.
Discussion
Allergic rhinitis is a disabling common disease. It represents a problem mainly in autumm and spring [1]. Symptomatic therapies have played an important role in its management because they are economic, fast and can be easily administered. In comparison therapies based on the pathophysiology, i.e. hyposensitization, have not offered clearcut benefits [1, 15]. Therefore, H1-receptor antagonists and sympathomimetic amines have been widely accepted for the treatment of this disorder [5]. The side effects of traditional H1-receptor antihistamines prevent their long term use, but the development of new drugs like loratadine, has largely overcome the problem, of central side effects [8, 16]. Pseudoephedrine, in combination with antihistamines in allergic rhinitis, potentiates the overall response, as has been shown in previous reports [11, 17, 18]. Considering that allergic rhinitis reaches a peak prevalence in childhood and adolescence, L+PS may be a therapeutic option for pediatrics patients.
There are few comparative trials on the efficacy of L+PS in allergic rhinitis [10–12, 18, 19]. All, but one, included both children and adults, so the effects in children may have been underestimated. Comparisons between L+PS and other antihistamines-pseudoephedrine combinations or placebo have suggested that L+PS is the best option. The exception is a study conducted by Paz Martinez in children which demonstrated that astemizole+pseudoephedrine was superior to L+PS [12]. Our global results (assessed by MTSS) have shown that L+PS is effective in treating the symptoms of allergic rhinitis, in accordance with previous studies. Interestingly, the significant changes from baseline observed in this study with both L+PS or PLA, were not reported by other trials. We have not compared L+PS with other combinations of antihistamines+pseudoephedrine, so the data reported in the Paz Martinez study [12] could not be confirmed. The lack of carryover effect shown here indicates that a washout period of 7 days is sufficient for these types of drugs.
Since each nasal endpoint was analysed separately, a clear distinction arose between symptoms and signs. While symptoms improved with both treatments, signs did only with L+PS. In previous reports [10–12, 14, 16–19] there was no clear division between subjective and objective manifestations nor any statistically significant change from baseline in individual factors. Authors have therefore used a ‘mean total symptom score’ in order to distinguish between treatments.
Our results demonstrated a positive placebo effect, particularly in symptoms, despite of previous data [14, 16, 18]. This could be explained in part by the intrinsic component (psychosomatic) of allergic responses, and in part by the type of measurement used. Frequently, psychosomatic responses are exacerbated in children due to the emotional overlay, thus an appropriate intervention by a physician can restrain allergic disorders. On the other hand, children's self-assessment of symptoms appear to be poorly reproducible; a recent paper [20] on this topic suggests that objective measurements should be preferred for this type of trial. Data shown here would support this.
Finally, one patient showed slight transient insomnia only when receiving L+PS, this adverse event may be attributable to the vasoconstrictor drug. We did not observe any rebound congestion during the washout period or during the 1 week follow up period after the end of the trial. The absence of changes in laboratory tests suggests that L+PS treatment was well tolerated. In conclusion, L+PS may be useful and safe in children with seasonal allergic rhinitis. However, elements like placebo effect, carryover and investigation of subjective symptoms/objective signs separately have not been sufficiently taken into account by previous trials. Their consideration would allow better planning of future trials.
Acknowledgments
We are indebted to Dr L. M. Zieher, Dr R. Iannantuono and BJCP's reviewers for their helpful criticism and comments, and to Ms C. Soria and Ms F. Capiaghi for their technical assistance.
References
- 1.Borge PA. Problems in allergic rhinitis. Arzneim Forsch Drug Res. 1982;32:1199–1201. [PubMed] [Google Scholar]
- 2.Wood RA, Doran TF. Atopic disease, rhinitis and conjunctivitis, and upper respiratory infections. Curr Opin Pediatr. 1995;7:615–627. doi: 10.1097/00008480-199510000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Nacleiro RM. The pathopysiology of allergic rhinitis: Impact of therapeutic intervention. J Allergy Clin Inmunol. 1988;82:927–934. [PubMed] [Google Scholar]
- 4.Foreman JC. Peptides and neurogenic inflammation. Br Med Bull. 1987;43:386–400. doi: 10.1093/oxfordjournals.bmb.a072189. [DOI] [PubMed] [Google Scholar]
- 5.Horak F. Seasonal allergic rhinitis: Newer treatment approaches. Drugs. 1993;45:518–527. doi: 10.2165/00003495-199345040-00004. [DOI] [PubMed] [Google Scholar]
- 6.Li CS, Hsu LY. Home dampness and childhood respiratory in subtropical climate. Arch Environ Health. 1996;51:42–46. doi: 10.1080/00039896.1996.9935992. [DOI] [PubMed] [Google Scholar]
- 7.Bener A, Galadari I, Naser KA. Pets, allergy and respiratory symptoms in children living in a desert country. Allerg Immunol Paris. 1995;27:190–195. [PubMed] [Google Scholar]
- 8.Clissold SP, Sorkin EM, Goa KL. Loratadine: A preliminary review of its pharmacodynamic properties and threapeutic efficacy. Drugs. 1989;37:42–57. doi: 10.2165/00003495-198937010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman BB, Lefkowitz RJ. Catecholamines, sympathomimetic drugs and adrenergic receptor antagonists. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. The Pharmacological Basis of Therapeutics. 9. New York: McGraw Hill Company; 1996. pp. 199–248. [Google Scholar]
- 10.Bronsky EA, Boggs P, Findlay S, et al. Comparative efficacy and safety of a once-daily loratadine-pseudoephedrine combination versus its components alone and placebo in the management of seasonal allergic rhinitis. J Allergy Clin Immunol. 1995;96:139–147. doi: 10.1016/s0091-6749(95)70001-3. [DOI] [PubMed] [Google Scholar]
- 11.Grossman J, Bronsky EA, Lainer BQ, et al. Loratadine-pseudoephedrine combination versus placebo in patients with seasonal allergic rhinitis. Ann Allergy. 1989;63:317–321. [PubMed] [Google Scholar]
- 12.Paz Martinez D, Rosales Parra E. Evaluación comparativa del astemizol-pseudoefedrina y loratadina-pseudoefedrina en niños con rinitis alérgica. Rev Alerg Mex. 1995;42:105–109. [PubMed] [Google Scholar]
- 13.Brody H. The lie that heals: The ethics of giving placebos. Ann Inter Med. 1982;97:112–118. doi: 10.7326/0003-4819-97-1-112. [DOI] [PubMed] [Google Scholar]
- 14.Dockhorn RJ, Bergner A, Connell JT, et al. Safety and efficacy of loratadine (Sch-29851): A new non-sedating antihistamine in seasonal allergic rhinitis. Ann Allergy. 1987;58:407–411. [PubMed] [Google Scholar]
- 15.Gallart T. Enfermedades alérgicas mediadas por anticuerpos IgE (hipersensibilidad inmediata o alergia atópica) In: Rozman C, García San Miguel J, Feliu E, editors. Medicina Interna. 12. Barcelona: Ediciones Doyma; 1992. pp. 2697–2713. [Google Scholar]
- 16.Gutkowsky A, Bedard P, Del Carpio J, et al. Comparison of the efficacy and safety of loratadine, terfenadine, and placebo in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 1988;81:902–907. doi: 10.1016/0091-6749(88)90948-7. [DOI] [PubMed] [Google Scholar]
- 17.Berkowitz RB, Connell JT, Dietz AJ, Greenstein SM, Tilkelman DG. The effectiveness of the non-sedating antihistamine loratadine plus pseudoephedrine in symptomatic management of the common cold. Ann Allergy. 1989;63:336–339. [PubMed] [Google Scholar]
- 18.Storms WW, Bodman SF, Nathan RA, et al. Sch-434. A new antihistamine/descongestant for seasonal allergic rhinitis. J Allergy Clin Immunol. 1989;83:1083–1090. doi: 10.1016/0091-6749(89)90450-8. [DOI] [PubMed] [Google Scholar]
- 19.Prevost M, Turenne Y, Moote DW, et al. Comparative study of Sch-434 and CTM-D in the treatment of seasonal allergic rhinitis. Clin Ther. 1994;16:50–56. [PubMed] [Google Scholar]
- 20.Watson WT, Roberts JR, Becker AB, Gendreau-Reid LF, Simons FE. Nasal patency in children with allergic rhinitis: Correlation of objective and subjective assessments. Ann Allergy Asthma Immunol. 1995;74:237–240. [PubMed] [Google Scholar]


