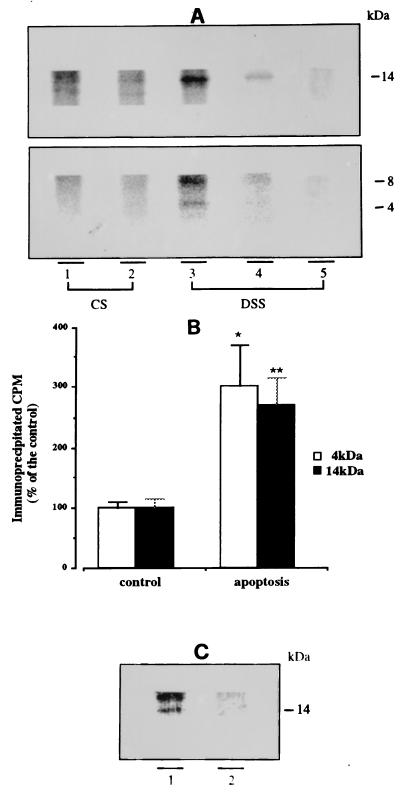
Figure 2.
Increased Aβ secretion in apoptotic neurons. After 7 days in vitro, cerebellar granule cells, metabolically prelabeled with [35S]methionine, were incubated for 6 h in 25 mM KCl/BME (controls) or 5 mM KCl/BME (apoptosis) to obtain CS medium. Both apoptotic and control neurons were subsequently incubated 30 min in 56 mM KCl Locke solution to obtain DSS medium. CS and DSS media were subjected to immunoprecipitation and the immunoprecipitates were separated on Tris-Tricine 10–18% gels. (A) Electrophoretic analysis of R3659-immunoprecipitated proteins. Lanes: 1, 3, and 5, from apoptotic neurons; 2 and 4, controls. Monomeric (4 kDa) and putative Aβ oligomers (8 kDa and 14 kDa) are visible only in DSS medium from apoptotic neurons (lane 3), whereas the same three bands are barely detectable in control DSS medium (lane 4). The 14-kDa oligomeric Aβ is also detectable in apoptotic CS medium. Preabsorption of the antibody with synthetic Aβ (lane 5) abolished the appearance of both mono- and oligomeric forms. (B) PhosphorImaging quantification of 4-kDa (open bars) and 14-kDa (solid bars) bands obtained from DSS medium, shown in A, lanes 3 and 4. Values relative to apoptotic neurons are expressed as percent of controls. Both 4-kDa and 14-kDa bands originating from apoptotic DSS contain three times the amount of radioactivity compared with controls. ∗, P < 0.002 (n = 4); ∗∗, P < 0.001 (n = 4). (C) Apoptotic (lane 1) and control (lane 2) DSS media were immunoprecipitated simultaneously with Aβ “flanking” antisera R1155 and R1872 (see text). The supernatant was further incubated with R3659 and the immunoprecipitated was analyzed by electrophoresis. Notice that the 14-kDa band is still present, but the 8-kDa peptide, probably isolated by one of the flanking antisera, is not visible.