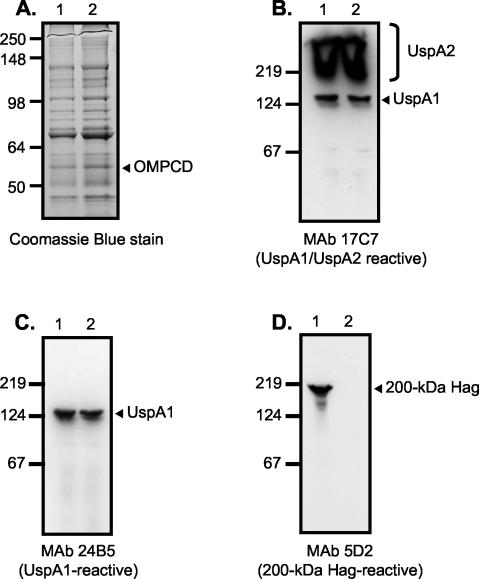
FIG. 2.
SDS-PAGE and Western blot analysis of M. catarrhalis parent strain O35E (lane 1 in all panels) and representative adherence-negative isolate O35E.TN2 (lane 2 in all panels). Proteins present in outer-membrane vesicles were resolved by SDS-PAGE and stained with Coomassie blue (A). Whole-cell lysates were prepared and analyzed by Western blotting with the UspA1- and UspA2-reactive MAb17C7 (B), the UspA1-specific MAb 24B5 (C), and the 200-kDa Hag-specific MAb 5D2 (D). Note that whole-cell lysates were heated at 100°C for 15 min prior to Western blot analysis. Under these conditions, UspA1 migrates as a 125-kDa antigen and UspA2 migrates as an aggregate of very high molecular weight that is expressed in large amounts (bracket on the right side of panel B) (12). The mutants appear to express wild-type levels of the OMPCD protein (arrow in panel A). Positions of molecular mass markers (in kilodaltons) are shown to the left of each panel.