FIG. 3.
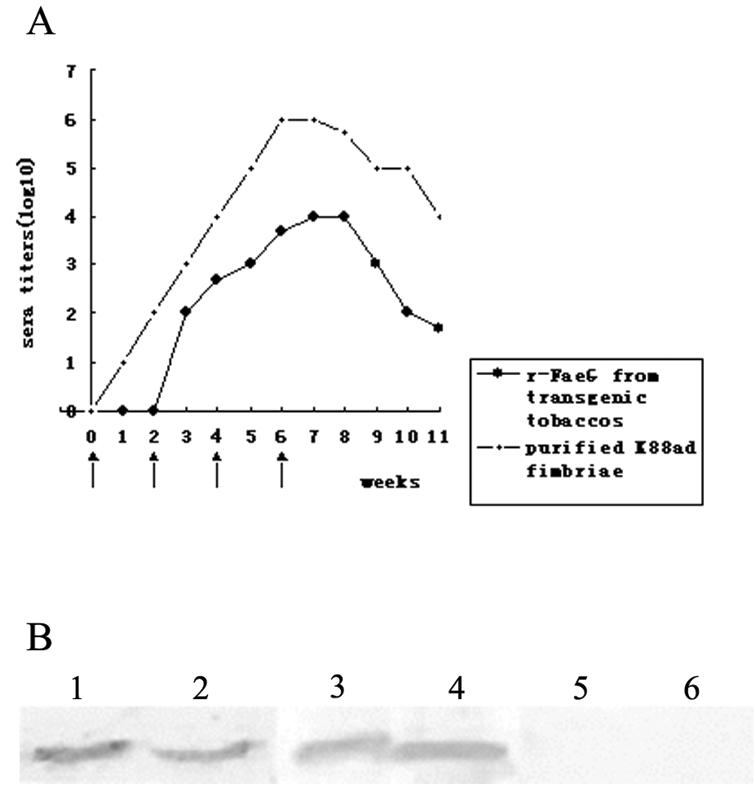
Immunogenicity of recombinant FaeG from transgenic tobacco. (A) Mice were immunized intraperitoneally on days 0, 14, 28, and 42 with either purified K88ad fimbriae or crude extracts from transgenic tobacco leaves. Arrows indicate the inoculating schedule. (B) Anti-FaeG antibodies detected by immunoblot analysis. ETEC K88ad fimbriae and purified recombinant FaeG from E. coli BL21(DE3+K88) were loaded in a well for sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. After the reaction mixtures were incubated with the sera of mice immunized with purified fimbriae, transgenic tobacco leaf extracts, or nontransgenic tobacco leaf extracts and then washed three more times, the reactions were developed by the addition of the substrate nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate. Lanes 1 and 2, serum from a mouse immunized with purified fimbriae as a positive control; lanes 3 and 4, serum from a mouse immunized with transgenic tobacco extracts; lanes 5 and 6, serum from a mouse immunized with nontransgenic tobacco extracts as a negative control. Lanes 1, 3, and 5, 0.5 μg of purified K88ad fimbriae; lanes 2, 4, and 6, 0.5 μg of purified recombinant FaeG from E. coli BL21(DE3+K88).
