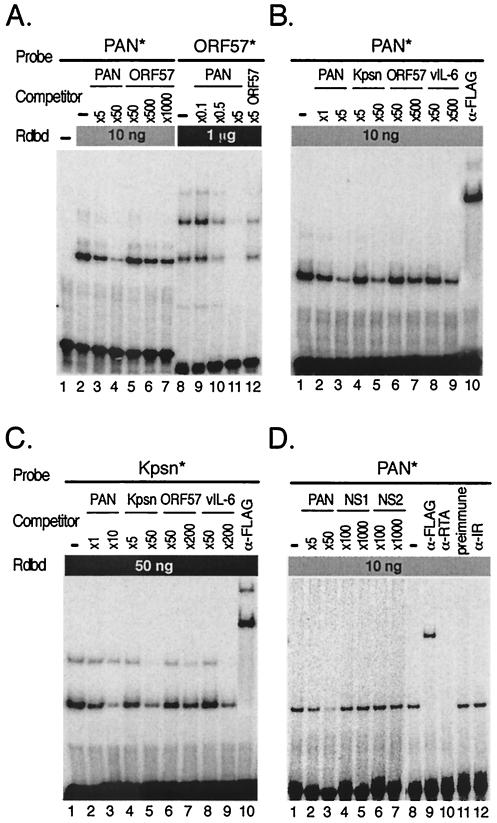
FIG. 7.
Cross-competition assays confirm relative affinities of Rdbd binding to the RREs. (A) Cross-competition assays between PAN and ORF57 RREs. Rdbd (10 ng) was incubated without (lane 2) or with excess unlabeled oligonucleotide, PAN (5- and 50-fold; lanes 3 and 4), or ORF57 (50-, 500-, and 1,000-fold; lanes 5 to 7), for competition assays prior to addition of PAN*. Indicated amounts of unlabeled oligonucleotide, PAN (0.1-, 0.5-, and 5-fold; lanes 9 to 11) or ORF57 (fivefold; lane 12), were mixed with 1 μg of Rdbd, followed by addition of ORF57*. Note that the amounts of the unlabeled oligonucleotide PAN used to compete for Rdbd binding were far less than those of the unlabeled oligonucleotide ORF57. (B) Cross-competition assays of PAN* (10 ng of Rdbd) were performed with a set of unlabeled oligonucleotides (PAN, Kpsn, ORF57, and vIL-6). As indicated, different amounts of cold competitors were used to produce similar levels of competition. Unlabeled oligonucleotides were preincubated with Rdbd, and an end-labeled probe, PAN* or Kpsn*, was added (lanes 2 to 9). A monoclonal antibody against the FLAG peptide was used to supershift protein-DNA complexes containing Rdbd (lanes 10). (C) Cross-competition assay of Kpsn* (50 ng of Rdbd) was performed as described for panel B. (D) Specificity of Rdbd binding to the RRE. The labeled PAN* probe were incubated in excess of unlabeled oligonucleotides NS1 and NS2, both of which contain nonspecific sequences, as negative controls. Coincubation of PAN* with unlabeled oligonucleotides containing its own sequence (PAN) resulted in efficient competition of the complex formation (lanes 2 and 3), while NS1 and NS2 did not show any significant competition (lanes 4 to 7). Supershift assays were performed with anti-FLAG antibody and polyclonal rabbit sera against RTA (lanes 9 and 10). Normal rabbit sera and polyclonal rabbit sera against irrelevant protein (lanes 11 and 12) were also included as negative controls.