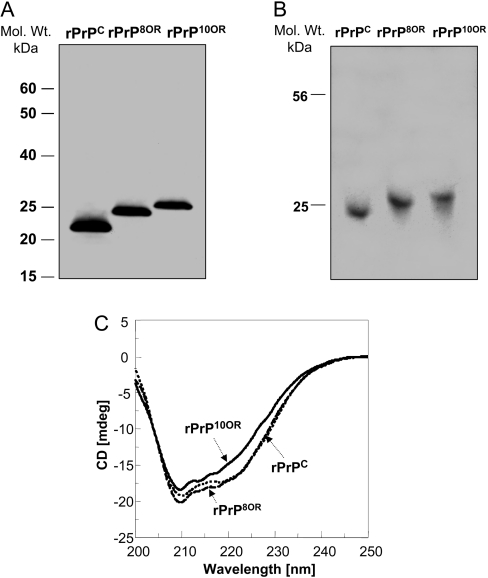
Figure 1. Characterization of rPrPC, rPrP8OR and rPrP10OR.
(A) Freshly prepared rPrPC, rPrP8OR and rPrP10OR proteins were separated by SDS/PAGE under non-reducing conditions and then immunoblotted with mAb 8H4. Molecular-mass (Mol. Wt.) markers are indicated in kDa to the left-hand side of the panel. (B) Acidic native gel electrophoresis of rPrPC, rPrP8OR and rPrP10OR. The gel was stained with Coomassie Blue. All three recombinant proteins exist as monomers under these conditions. (C) Far-UV CD spectra of rPrPC, rPrP8OR and rPrP10OR. The spectra were obtained using 20 μM of rPrPC (dotted line), rPrP8OR (broken line) and rPrP10OR (solid line) in 20 mM sodium acetate (pH 5.5). While rPrPC and rPrP8OR have rather similar secondary structures, rPrP10OR has a small decrease in the negativity of the spectra. Therefore insertion mutations at the N-terminus are able to influence the secondary structure of the molecule, depending on the number of octapeptide-repeat inserts.