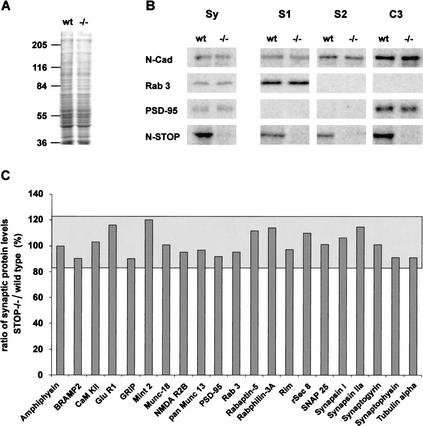
Figure 4.
Biochemical composition of synaptosomal fractions in STOP−/− mice. (A) Coomassie blue-stained SDS-PAGE of synaptosomal fractions from wild-type and STOP−/− mice. No obvious difference in protein content was found between the two fractions. Total synaptosomal proteins (Sy) and fractions S1, S2, and C3 from wild-type and STOP−/− mice (see Materials and Methods) were analyzed by quantitative immunoblotting with 125I-labeled secondary antibody and phosphorimager detection. (B) Representative immunoblots of synaptosomal proteins. Equal amounts of protein from fractions Sy and equal volumes of each fraction, S1, S2, and C3 from wild-type and STOP−/− mice were analyzed on the same immunoblot. With this procedure, signal intensities in fractions S1, S2, and C3 reflect the distribution of proteins among these fractions. In both phenotypes, protein profiles were similar (except for N-STOP). N-Cadherin, a pre- and postsynaptic protein, was found in all fractions; Rab3, a presynaptic protein, was found only in S1 fraction, and PSD-95, a postsynaptic protein tightly associated with the postsynaptic density, was found in the insoluble fraction C3. N-STOP protein was found in all fractions with a higher concentration in fraction C3. (C) Synaptic proteins profiles. The ratios of synaptic protein levels in STOP−/− vs. wild-type were plotted (n = 2). Results are shown for fraction Sy. The limits of the gray area corresponds to the 95% confidence interval, determined by ANOVA. All observed ratios were within this confidence interval. Similar analysis with fractions S1, S2, and C3 showed no significant difference (data not shown).