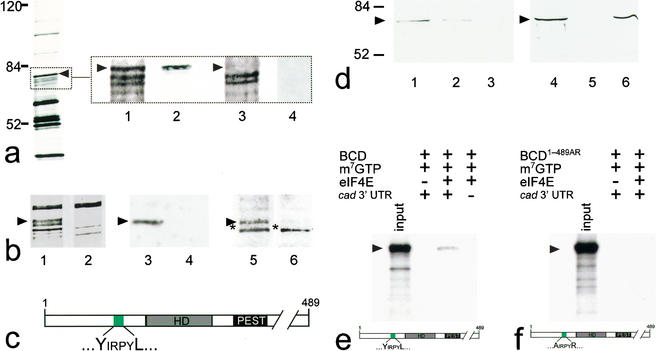
Figure 1.
BCD copurifies with 5′-cap-bound proteins. Cytoplasmic protein extracts of young embryos were affinity-purified using a cap-analog m7GTP-sepharose resin (Edery et al. 1988). (a) Silver-stained SDS-PAGE of affinity-purified proteins contained within cytoplasmic extracts of wild-type and bcd mutant Drosophila embryos. Arrowhead marks the protein band that was subsequently identified as BCD (left, see also lanes 1–4 in b). Note that eIF4E, the most abundant 30-kD component among the purified proteins, is run off the gel (left) to obtain maximum resolution of the relevant range of protein bands between 50 and 100 kD. (Lanes 1–4) Relevant portions of silver-stained SDS-PAGE (dotted box) including a ∼70-kD protein (lane 1, arrowhead) that is recognized by monospecific anti-BCD antibodies on Western blots (lane 2). This protein is not present in silver-stained SDS-PAGE (lane 3), and anti-BCD antibodies stained Western blots (lane 4) of corresponding extracts from embryos that derived from homozygous bcd mutant females. (b) Relevant portions of silver-stained SDS-PAGE showing that the GFP–BCD fusion protein (lane 1, 95 kD) is absent in wild-type embryos (lane 2). Corresponding Western blots show that the 95-kD fusion protein reacts with both anti-BCD (lane 3; lane 4 is a wild-type control lacking the GFP–BCD transgene) and anti-GFP antibodies (lane 5; lane 6 is a wild-type control lacking the GFP–BCD transgene; asterisk marks cross-reacting protein of unknown identity). (c) Schematic diagram of BCD (positions refer to sequence according to Berleth et al. 1988) showing the homeodomain (HD, gray box; position 91–154), the PEST domain (PEST, black box; position 170–203), and a YIRPYL motif (green box; position 68–73). The eIF4E-binding properties of this motif have been recently analyzed in great detail in the context of human BP1 (Marcotrigiano et al. 1999; for review, see Raught et al. 2000; Sachs and Varani 2000; Miron et al. 2001). (d) Western blots showing that BCD bound to m7GTP-sepharose coupled recombinant eIF4E (lanes 1,4) can be competed for with 100 nM (lane 2) and 1 mM (lanes 3,5) of a peptide containing the YDRKFL motif of human BP1, whereas 1 mM of a mutated human BP1 peptide (mutated motif is ADRKFR) did not interfere with the binding of BCD to eIF4E (lane 6). (e,f) Autoradiogram showing that in vitro translated 35S-labeled BCD (input; a schematic representation of the protein is shown at the bottom of e) is capable of interacting with m7GTP-sepharose-bound recombinant eIF4E in the presence of in vitro transcribed cad 3′-UTR (e), whereas in vitro translated 35S-labeled BCD1–489AR (input; a schematic representation of the protein is shown at the bottom of f) showed no significant binding to eIF4E (f). Note that there is no unspecific binding of in vitro translated protein to m7GTP-beads without precoupled eIF4E. For details, see Materials and Methods.