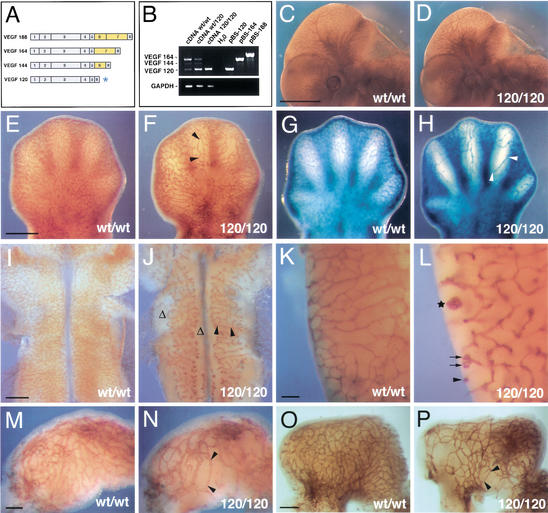
Figure 1.
Loss of heparin-binding VEGF-A reduces vascular branching complexity during embryogenesis. (A) VEGF-A isoforms in the mouse; the isoforms differ by the absence or presence of heparin-binding domains encoded by exons 6 and 7 (highlighted in yellow); the VEGF120 isoform (asterisk) is the only isoform expressed when exons 6 and 7 are ablated. (B) RT-PCR of cDNA derived from 12.5-dpc hindbrains (wt/wt, wt/120, and 120/120) using oligonucleotides specific for VEGF-A or GAPDH; cloned cDNAs (pBS-120, pBS-164, and pBS-188) were used as controls. (C,D) Whole-mount immunohistochemistry of wt/wt (C) and 120/120 (D) littermate embryos at 11.25 dpc using an anti-PECAM mAb shows that loss of heparin-binding VEGF-A does not ablate vascularization during embryogenesis. (E–H) Vascular networks in the limb at 12.5 dpc (E,F) and 13.5 dpc (G,H). (I–L) Vascular networks in the hindbrain at 11.25 dpc (I,J) and in the posterior lateral hindbrain at 13.5 dpc (K,L). (M–P) Vascular networks in the stomach at 11.25 dpc (M,N) and 12.5 dpc (O,P). Blood vessels in stage matched wt/wt (C,E,G,I,K,M,O) and 120/120 (D,F,H,J,L,N,P) littermates were visualized by immunohistochemistry using an antibody to PECAM (C–F,I–P) or a Tie2LacZ reporter (G,H). Examples of vessel abnormalities are highlighted. Stretches devoid of branchpoints are labeled with black arrowheads; regions severely impaired in branching are indicated with Δ; vessel branches with coiling ends are labeled with black arrows; and a large vessel tuft is with a star. Bars: C,D, 1 mm; E–H, 500 μm; I,J, 500 μm; K,L, 100 μm; M,N, 100 μm; O,P, 200 μm.