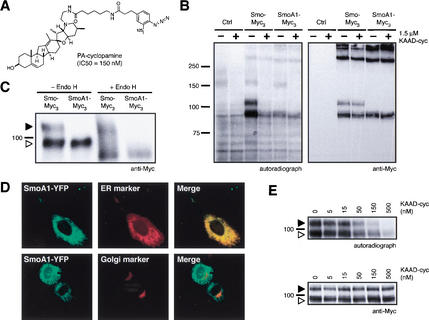
Figure 1.
A photoaffinity derivative of cyclopamine cross-links Smo. (A) Chemical structure of PA-cyclopamine and its inhibitory activity on Shh signaling. (B) Upon photoactivation, 125I-labeled PA-cyclopamine cross-links two forms of Smo fused at the C terminus to Myc epitopes (Smo–Myc3) in COS-1 cells, and this labeling is inhibited by 1.5 μM KAAD-cyclopamine (left panel). Nontransfected cells and SmoA1–Myc3-expressing cells do not yield specifically cross-linked products. Western analysis with an anti-Myc antibody demonstrates that Smo–Myc3 and SmoA1–Myc3 expression levels are comparable and are not affected by KAAD-cyclopamine treatment (right panel). (C) The two Smo–Myc3 forms represent different glycosylation states, as one is endo H-sensitive (open arrowhead) and the other endo H-resistant (solid arrowhead). SmoA1–Myc3 is exclusively observed as an endo H-sensitive form. Phosphatase treatment did not alter the mobilities of Smo–Myc3 or SmoA1–Myc3 proteins (data not shown). (D) Endo H-sensitivity is indicative of ER localization, as confirmed by the colocalization of SmoA1–YFP (pseudocolored green, top left panel) and an ER marker (pseudocolored red, top middle panel; merge, top right panel) in C3H/10T1/2 cells. Cells expressing both SmoA1–YFP (pseudocolored green, bottom left panel) and a Golgi marker (pseudocolored red, bottom middle panel) exhibit no colocalization (merge, bottom right panel). (E) KAAD-cyclopamine abrogates Smo–Myc3/PA-cyclopamine cross-linking in a manner that is consistent with its inhibitory activity in the Shh-LIGHT2 assay (left panel) without altering cellular levels of Smo–Myc3 (right panel). Both ER and post-ER forms of Smo–Myc3 are depicted as described above. Cross-linking of an endogenous 160-kD protein (B) was competed by KAAD-cyclopamine only at concentrations significantly higher than those required for pathway inhibition (data not shown).