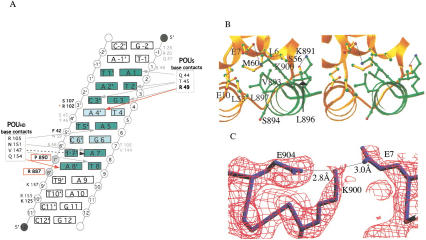
Figure 3.
SNAP190 interacts with both the Oct-1 POUS domain and U1 octamer element. (A) Schematic representation of the protein/DNA contacts within the SNAP190/Oct-1/U1 octamer complex. POUS is numbered beginning with the N terminus of the domain, whereas POUHD begins at 101. The octamer bases are green. Lighter green highlights the differences between the U1 and H2B octamers. Red arrows indicate contacts not observed in the structure of Oct-1 POU alone. R49 (bold) makes a contact unique in SNAP190, whereas the Q154 contact is observed in both the OCA-B (Chasman et al. 1999) and SNAP190 structures. Gray arrows indicate contacts observed in all three Oct-1 POU-domain structures. The black dashed arrow indicates a base-specific contact shared only in the Oct-1 POU alone and SNAP190 structures. SNAP190/DNA phosphate backbone contacts (R887, P890) are in bold and boxed in red. S107 and F42 (bold) make phosphate backbone contacts that are observed in both the SNAP190 and OCA-B structures, whereas R102 (red asterisk) is seen only in the SNAP190 structure. The backbone contacts by K125 and K157 (dark gray type) are observed in the Oct-1/OCA-B/DNA and Oct-1/U1 DNA/SNAP190 structures. Amino acids within the POU domain that participate in phosphate backbone contacts observed in all three structures are shown in gray type. (B) A stereo view of the POUS (gold) interaction with SNAP190 (green). (C) A simulated annealing omit map contoured at 1.8 ς around SNAP190 K900, POUS E7, and SNAP190 E904. SNAP190 E904 buttresses K900, accurately positioning it to make a critical salt bridge with POUS E7 (Evans 1993).