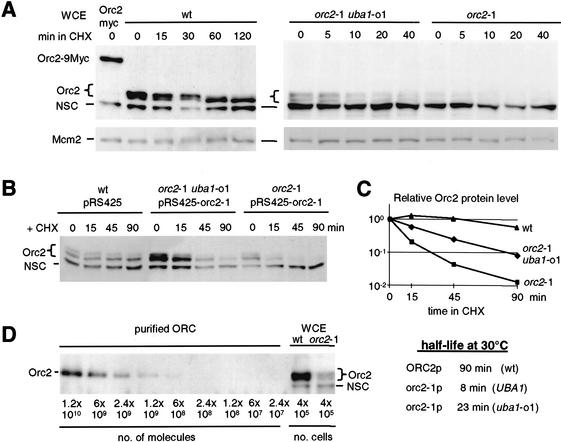
Figure 3.
The highly labile protein orc2-1p is partially stabilized by the uba1-o1 mutation. (A) Cycloheximide was added to exponentially growing cells and total protein extracts were prepared at indicated times. Western blots were sequentially probed with goat anti-MCM2 (Santa Cruz Antibodies) or affinity-purified anti-Orc2p antibody. The brace indicates to a doublet of Orc2p-specific bands (the upper band being phosphorylated); NSC indicates a nonspecific cross-reaction with a cytoplasmic protein that serves as an internal control for loading. Strains used were orc27∷ORC2-9Myc (GA-893); wild-type (GA-180); orc2-1 uba1-o1 (GA-463); orc2-1 (GA-1254). (B) Isogenic orc2-1 and orc2-1 uba1-o1 cells transformed with a multicopy plasmid expressing mutant orc2-1p (pRS425-orc2-1) and wild-type cells with the empty vector, were treated with cycloheximide (CHX) at 30°C, and whole-cell extracts were prepared at the indicated times. Orc2p protein was revealed as in A. (C) Orc2p protein signals in B were quantified by the AIDA program (Fuji PhosPhorimager) and normalized to the lower nonspecific band (NSC). Relative intensity is plotted as a function of time after CHX addition and the half-life of Orc2p (t1/2) was calculated. (D) Decreasing amounts of purified recombinant Baculovirus-expressed yeast ORC complex (gift of Dr. S. Bell), and total protein extracts equivalent to 4 × 105 cells from isogenic wild-type and orc2-1 cells grown at 23°C, were analyzed by Western blot using affinity-purified anti-Orc2 antibodies.