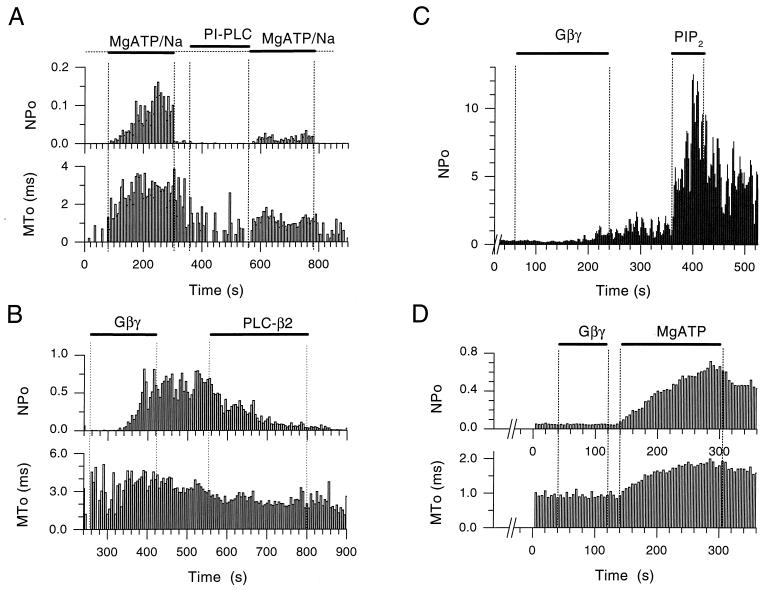
Figure 3.
Depletion of PIP2 by PLCs blocks MgATP and Gβγ stimulation of KACh channel activity and restoration of PIP2 reverses these effects. (A) Single-channel NPo and MTo plots as a function of time in the experiment were obtained from an inside-out patch of a Xenopus oocyte expressing GIRK1/GIRK4. The KACh channels were first activated by application of MgATP/Na (5/20 mM). Both NPo and MTo were increased by MgATP/Na. Subsequent treatment of the inside-out patch with phosphatidylinositol-specific PLC (1 unit/ml) for approximately 2.5 min, significantly reduced activation by MgATP/Na, causing a concomitant decrease in MTo. (B) Single-channel NPo and MTo plots, obtained from an inside-out patch of a Xenopus oocyte expressing GIRK1/GIRK4. Soon after excision, Gβγ application (20 nM) caused persistent channel activation. Subsequent treatment of the patch with PLC-β2 (5 μg/ml) inhibited activity with a concomitant decrease in MTo. (C) NPo plot as a function of time in the experiment from an inside-out patch of Xenopus oocyte expressing GIRK1/GIRK4. The patch was exposed to PLC-β2 (5 μg/ml) for a period greater than 5 min, before Gβγ (20 nM) application. PLC-β2 treatment greatly retarded Gβγ effectiveness. Subsequent perfusion with PIP2 (5 μM) revealed high channel activity, thus rescuing the Gβγ action. The number of active KAch channels in the membrane was greater than 5, thus precluding analysis of MTo [see Sui et al. (13)]. (D) Single-channel NPo and MTo plots, obtained from an inside-out patch of Xenopus oocyte expressing GIRK1/GIRK4. Perfusion of the patch with ATP-free solutions for several minutes rendered Gβγ (20 nM) ineffective. Subsequent addition of MgATP (5 mM) to the membrane revealed high channel activity, thus rescuing the Gβγ action, which was presumably still bound but ineffective.