Abstract
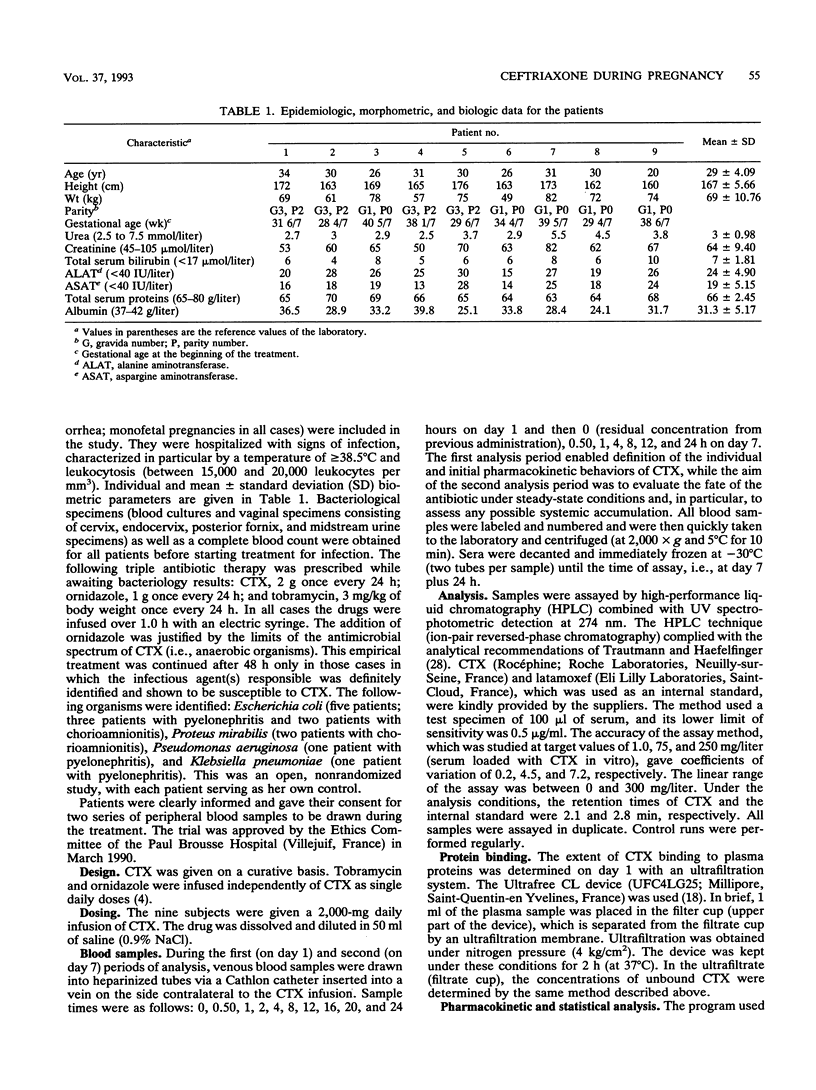
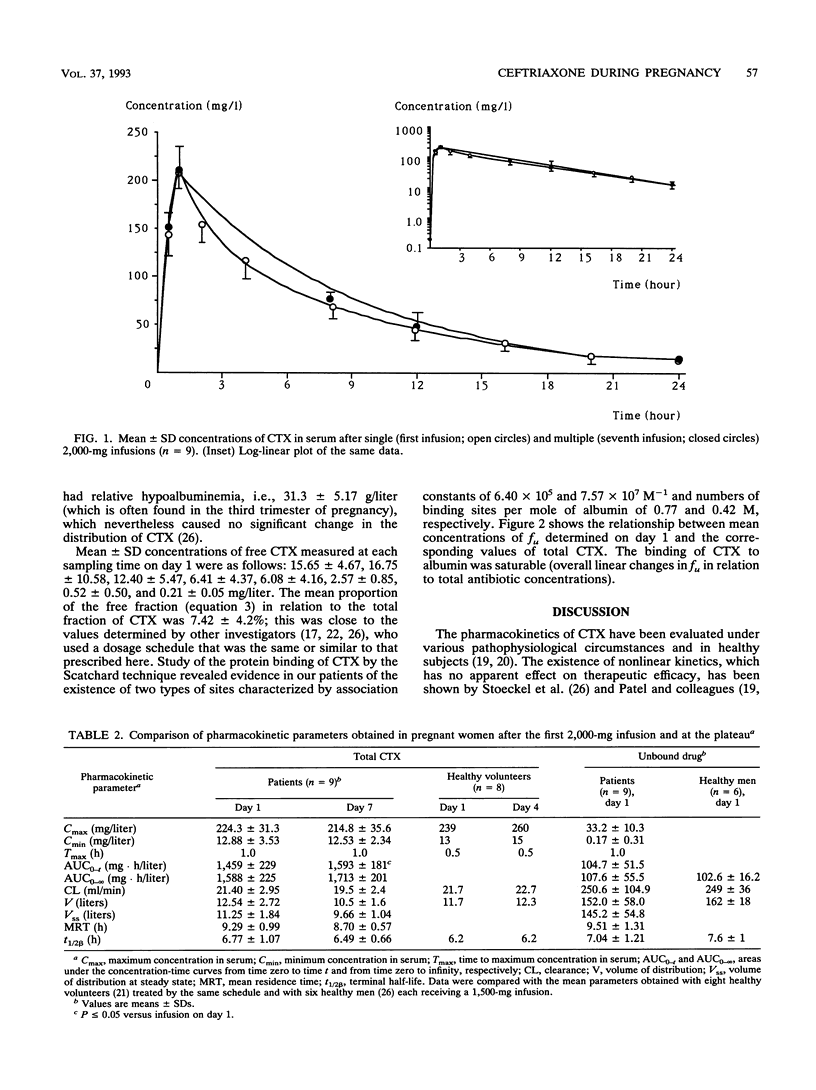
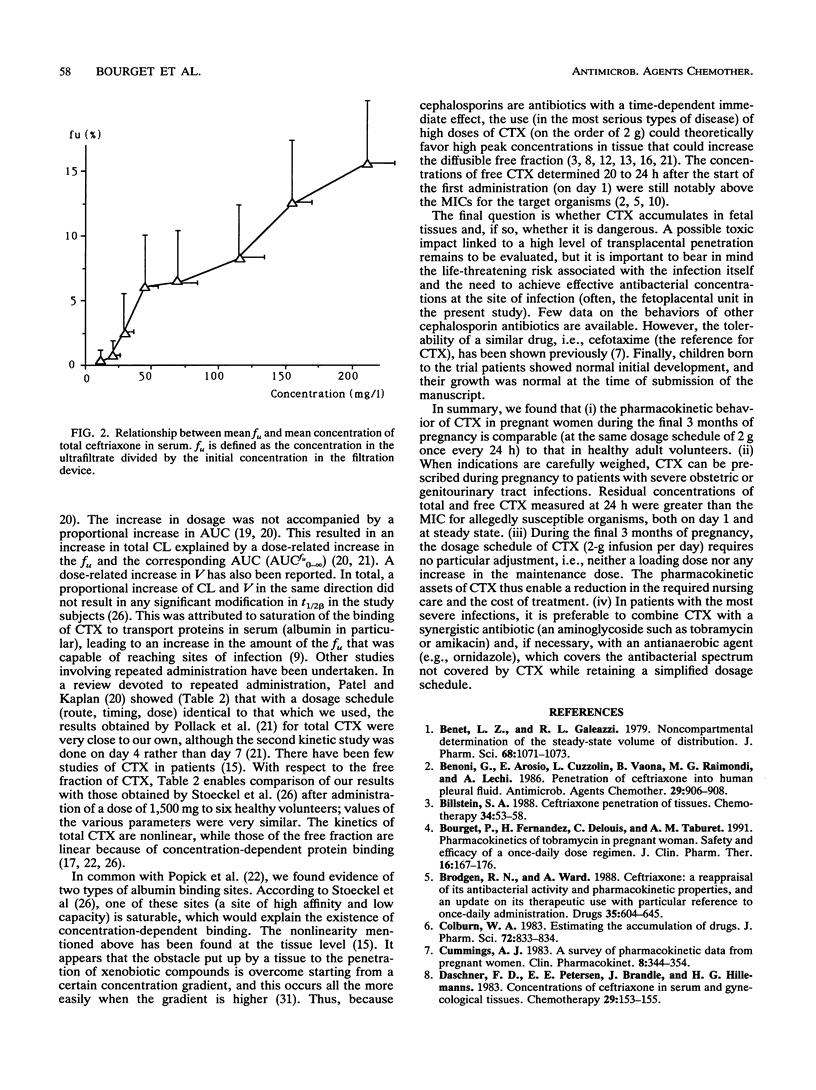
The purpose of the present work was to study the pharmacokinetics and the protein binding (free fraction of the drug) of ceftriaxone (CTX) during pregnancy. Nine pregnant women (ages, 20 to 34 years) whose gestational ages ranged from 28 4/7 to 40 5/7 weeks were included. The diagnosis of infection was established in all cases; i.e., four women had chorioamnionitis and five women had pyelonephritis. The following triple antibiotic therapy was infused with the aim of achieving cure: CTX, 2 g once every 24 h (constant rate over 60 min); tobramycin, 3 mg/kg of body weight once every 24 h; and ornidazole, 1 g/day. Two series of blood samples were collected, i.e., during the first day of treatment (on day 1), to establish the primary pharmacokinetic profile of CTX, and at the plateau (on day 7), to evaluate a possible accumulation of the drug. This was an open, noncompartmental study, with each patient serving as her own control. Concentrations of total and unbound CTX in serum were measured by a high-performance liquid chromatographic method. Pharmacokinetic analysis was done by a noncompartmental method. Data were compared by a Wilcoxon t test (a P value of < or = 0.05 was considered significant). Data were also compared with those obtained for healthy subjects who received similar treatments. (i) The tolerance to treatment was excellent, and in all cases patients had a complete remission without premature delivery. (ii) No accumulation of CTX was noted during the treatment, and the profiles of the drug determined at days 1 and 7 were not significantly different.(iii) The pharmacokinetic parameters measured in pregnant patients during the third trimester of pregnancy were similar to those measured in healthy subjects. (iv) Residual concentrations of total and unbound CTX measured at 24 h were greater than the MICs for allegedly susceptible organisms, both on day 1 and at steady state. (v) During the final 3 months of pregnancy, the dosage schedule of CTX (2-g infusion per day) required no particular adjustment (i.e., neither a loading dose nor any increase in the maintenance dose.)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Benoni G., Arosio E., Cuzzolin L., Vaona B., Raimondi M. G., Lechi A. Penetration of ceftriaxone into human pleural fluid. Antimicrob Agents Chemother. 1986 May;29(5):906–908. doi: 10.1128/aac.29.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billstein S. A. Ceftriaxone penetration of tissues. Chemotherapy. 1988;34 (Suppl 1):53–58. doi: 10.1159/000238648. [DOI] [PubMed] [Google Scholar]
- Bourget P., Fernandez H., Delouis C., Taburet A. M. Pharmacokinetics of tobramycin in pregnant women. Safety and efficacy of a once-daily dose regimen. J Clin Pharm Ther. 1991 Jun;16(3):167–176. doi: 10.1111/j.1365-2710.1991.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Brogden R. N., Ward A. Ceftriaxone. A reappraisal of its antibacterial activity and pharmacokinetic properties, and an update on its therapeutic use with particular reference to once-daily administration. Drugs. 1988 Jun;35(6):604–645. doi: 10.2165/00003495-198835060-00002. [DOI] [PubMed] [Google Scholar]
- Colburn W. A. Estimating the accumulation of drugs. J Pharm Sci. 1983 Jul;72(7):833–834. doi: 10.1002/jps.2600720734. [DOI] [PubMed] [Google Scholar]
- Cummings A. J. A survey of pharmacokinetic data from pregnant women. Clin Pharmacokinet. 1983 Jul-Aug;8(4):344–354. doi: 10.2165/00003088-198308040-00005. [DOI] [PubMed] [Google Scholar]
- Daschner F. D., Petersen E. E., Brändle J., Hillemanns H. G. Concentrations of ceftriaxone in serum and gynecological tissues. Chemotherapy. 1983;29(2):153–155. doi: 10.1159/000238189. [DOI] [PubMed] [Google Scholar]
- De Grandi P., Bauen J. F. Pharmacokinetics of ceftriaxone in serum and gynecological tissues. Chemotherapy. 1986;32(6):473–477. doi: 10.1159/000238455. [DOI] [PubMed] [Google Scholar]
- Deforges L., Le Van Thoi J., Soussy C. J., Duval J. Activité cantibactérienne in vitro de huit céphalosporines de troisième génération. Pathol Biol (Paris) 1982 Jun;30(6):363–369. [PubMed] [Google Scholar]
- Fraschini F., Braga P. C., Scarpazza G., Scaglione F., Pignataro O., Sambataro G., Mariani C., Roviaro G. C., Varoli F., Esposti G. Human pharmacokinetics and distribution in various tissues of ceftriaxone. Chemotherapy. 1986;32(3):192–199. doi: 10.1159/000238415. [DOI] [PubMed] [Google Scholar]
- Hemsell D. L., Menon M. O., Friedman A. J. Ceftriaxone or cefazolin prophylaxis for the prevention of infection after vaginal hysterectomy. Am J Surg. 1984 Oct 19;148(4A):22–26. [PubMed] [Google Scholar]
- Joos B., Luethy R., Muehlen E., Siegenthaler W. Variability of ceftriaxone pharmacokinetics in hospitalized patients with severe infections. Am J Med. 1984 Oct 19;77(4C):59–62. [PubMed] [Google Scholar]
- Kafetzis D. A., Brater D. C., Fanourgakis J. E., Voyatzis J., Georgakopoulos P. Ceftriaxone distribution between maternal blood and fetal blood and tissues at parturition and between blood and milk postpartum. Antimicrob Agents Chemother. 1983 Jun;23(6):870–873. doi: 10.1128/aac.23.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luderer J. R., Patel I. H., Durkin J., Schneck D. W. Age and ceftriaxone kinetics. Clin Pharmacol Ther. 1984 Jan;35(1):19–25. doi: 10.1038/clpt.1984.3. [DOI] [PubMed] [Google Scholar]
- McNamara P. J., Stoeckel K., Ziegler W. H. Pharmacokinetics of ceftriaxone following intravenous administration of a 3 g dose. Eur J Clin Pharmacol. 1982;22(1):71–75. doi: 10.1007/BF00606428. [DOI] [PubMed] [Google Scholar]
- McNamara P. J., Trueb V., Stoeckel K. Protein binding of ceftriaxone in extravascular fluids. J Pharm Sci. 1988 May;77(5):401–404. doi: 10.1002/jps.2600770509. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Johno I., Kitazawa S. Comparative evaluation of microultrafiltration devices for determination of protein binding. Ther Drug Monit. 1988;10(3):310–315. doi: 10.1097/00007691-198803000-00013. [DOI] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Kaplan S. A. Pharmacokinetic profile of ceftriaxone in man. Am J Med. 1984 Oct 19;77(4C):17–25. [PubMed] [Google Scholar]
- Pollock A. A., Tee P. E., Patel I. H., Spicehandler J., Simberkoff M. S., Rahal J. J., Jr Pharmacokinetic characteristics of intravenous ceftriaxone in normal adults. Antimicrob Agents Chemother. 1982 Nov;22(5):816–823. doi: 10.1128/aac.22.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popick A. C., Crouthamel W. G., Bekersky I. Plasma protein binding of ceftriaxone. Xenobiotica. 1987 Oct;17(10):1139–1145. doi: 10.3109/00498258709167406. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Koup J. R. Pharmacokinetics of ceftriaxone in patients with renal and liver insufficiency and correlations with a physiologic nonlinear protein binding model. Am J Med. 1984 Oct 19;77(4C):26–32. [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- Stoeckel K. Pharmacokinetics of Rocephin, a highly active new cephalosporin with an exceptionally long biological half-life. Chemotherapy. 1981;27 (Suppl 1):42–46. doi: 10.1159/000238028. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Tuerk H., Trueb V., McNamara P. J. Single-dose ceftriaxone kinetics in liver insufficiency. Clin Pharmacol Ther. 1984 Oct;36(4):500–509. doi: 10.1038/clpt.1984.210. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978 Dec;6(6):547–558. doi: 10.1007/BF01062109. [DOI] [PubMed] [Google Scholar]
- Yuk J. H., Nightingale C. H., Quintiliani R. Clinical pharmacokinetics of ceftriaxone. Clin Pharmacokinet. 1989 Oct;17(4):223–235. doi: 10.2165/00003088-198917040-00002. [DOI] [PubMed] [Google Scholar]


