ABSTRACT
This report is a retrospective analysis of the surgical outcome of 15 patients (8 females, 7 males; mean age, 37.8 years) with residual or recurrent vestibular schwannomas operated on between 1987 and 2005. These 15 patients were part of a larger series of 252 consecutive vestibular schwannoma excisions. Tumors were classified as large (10) when their diameter exceeded 3.5 cm and giant (5) when their diameter exceeded 4.5 cm. All patients had previously undergone surgery. Hearing was lost in all cases, 8 had complete facial nerve palsy, 6 had trigeminal nerve deficits, 5 had cranial nerve IX and X palsy, and 10 had ataxic gait. Twelve patients had hydrocephalus. The tumors were reoperated through the retrosigmoid-transmeatal approach. The mean postoperative follow-up was 4.9 years. Complete resection was achieved in all patients. The facial nerve was preserved in 6 of the 7 patients with preoperative facial function. Transient worsening of bulbar cranial nerves palsy occurred in 2 cases. Cerebrospinal fluid leakage occurred in 3 patients. There were no deaths, and the tumors were histologically benign. Surgical removal is the only treatment for these lesions. Total resection associated with a low morbidity rate is possible. Preservation of the facial nerve is difficult due to severe scar tissue.
Keywords: Vestibular schwannomas, residual acoustic neurinomas, recurrent acoustic neurinomas, giant acoustic neurinoma, surgery
With the advent of microsurgery and better diagnostic procedures, safe total resection of vestibular schwannomas (VS) has become routine. Almost all tumors can be removed totally with few complications. Facial nerve function, and, in selected cases, cochlear nerve function can be preserved. Occasionally, however, total resection may be not possible even when the operation is performed by the most experienced surgeon. Small recurrences are usually treated by surgery or stereotactic radiosurgery, and the outcomes are very good.1,2,3,4 Series of small and medium-size residual and recurrent VS have been reported.1,3,4,5 Large and giant residual and recurrent VS compressing the brainstem are rare because most patients undergoing subtotal resection are closely followed with radiological studies to detect further growth of the residual tumor. Treatment of these large lesions is complex and difficult. In fact, no studies on the clinical features and surgical outcomes of patients with such VS could be found in the literature. We therefore evaluated 15 patients with large and giant residual or recurrent VS treated with radical surgery.
MATERIAL AND METHODS
From 1987 to 2005, 252 patients with VS were treated surgically by the senior author (RR). Of these, 21 patients presented with residual or recurrent tumors. Nineteen cases underwent previous surgery elsewhere, and 15 had large or giant tumors. Two patients with small intracanalicular residual tumors had previously been treated surgically at our clinic (1 with a large cystic schwannoma and 1 with a 2-cm tumor); therefore, the recurrence rate was 0.8% (2/252). Both schwannomas were totally removed (with hearing preservation in the patient with the small tumor), as confirmed by postoperative magnetic resonance imaging (MRI). These tumors recurred 2 and 3.5 years, respectively, after surgery. Tumors were classified as large when their diameter exceeded 3.5 cm and as giant when their diameter exceeded 4.5 cm and caused marked brainstem compression. Ten VS were classified as giant and 5 as large (Table 1).
Table 1.
Patients with Large and Giant Residual or Recurrent Vestibular Schwannomas
| Patient | Sex/Age | Number of Previous Surgeries | Size 1† (cm) | Type | Resection (%) | Radiation* | Size 2†† (cm) | Time** (years) |
|---|---|---|---|---|---|---|---|---|
| CSR, conformational stereotactic radiotherapy; GKS, gamma knife surgery. | ||||||||
| 1 | F/23 | 1 | 4.0 | Solid | 40 | No | 4.5 | 3 |
| 2 | M/27 | 2 | 4.0 | Cystic | 30 | No | 5.0 | 2 |
| 3 | F/58 | 1 | 4.0 | Solid | 40 | No | 3.5 | 5 |
| 4 | M/21 | 1 | 4.5 | Solid | 30 | No | 4.5 | 1.5 |
| 5 | M/29 | 3 | 5.0 | Cystic | 60 | No | 5.0 | 3 |
| 6 | M/52 | 2 | 4.5 | Solid | 40 | No | 4.0 | 4 |
| 7 | F/38 | 1 | 4.0 | Solid | 30 | No | 4.0 | 3 |
| 8 | M/42 | 3 | 4.0 | Solid | 40 | No | 4.5 | 2.5 |
| 9 | F/47 | 1 | 4.0 | Solid | 40 | No | 4.0 | 2 |
| 10 | F/40 | 2 | 4.5 | Solid | 30 | CSR | 4.5 | 2 |
| 11 | F/35 | 2 | 5.0 | Solid | 50 | No | 5.0 | 4 |
| 12 | F/47 | 2 | 4.0 | Cystic | 50 | GKS | 5.0 | 3.5 |
| 13 | M/28 | 2 | 4.5 | Cystic | 30 | No | 4.5 | 1.5 |
| 14 | F/39 | 2 | 4.0 | Solid | 50 | No | 4.5 | 4 |
| 15 | M/42 | 1 | 3.5 | Solid | 30 | GKS | 4.0 | 2.5 |
Size at the first surgery.
Size before definitive surgery.
Radiation therapy after previous surgery/ies.
Time between last surgery and definitive surgery.
Eight patients were female and 7 were male (mean age, 37.8 years; range, 21 to 58 years). The number of previous surgeries was one in 6 cases, two in 7 cases, and three in 2 cases. Ten patients presented with ataxia. Hearing was lost in all patients. Eight presented with complete facial nerve palsy, 6 had facial numbness, and 5 had a hoarse voice and swallowing difficulties. Tracheotomy and gastrostomy had been performed in 1 case after two surgeries and conformational stereotactic radiotherapy. A shunt was placed to relieve hydrocephalus in 12 patients. Gamma knife radiosurgery had been performed in 2 cases and conformational radiotherapy in 1 case. Despite these treatments, these cases showed further tumor growth.
All tumors had been partially resected during the previous surgery/ies. Tumor size at first surgery varied from 3.5 to 5.0 cm, and four were cystic (Table 1). The mean time between the last surgery and the definitive surgery in our department was 2.8 years (range, 1.5 to 5 years). According to the previous surgeons, extensive bleeding, adherences to brainstem and facial nerve, and cerebellar edema were the causes of partial removal. The internal auditory canal (IAC) was drilled during the previous surgery/ies in five cases.
The retrosigmoid-transmeatal approach was used to remove the recurrent or residual VS in all 15 cases. Surgery was performed with patients in the dorsal (mastoid) position with the head rotated to the contralateral side and the shoulder elevated. Intraoperative facial nerve monitoring was performed in patients with preoperative facial nerve function. The mean postoperative follow-up was 4.9 years (range, 4 months to 10 years).
RESULTS
Total tumor removal was accomplished in all 15 cases (Table 2). Dissection of the dura and identification of the sigmoid sinus were difficult due to fibrosis. The arachnoidal plane was poorly defined, and careful dissection of the tumor capsule from the vessels, nerves, and brainstem was challenging. Scar tissue causing severe adhesions was the main surgical difficulty.
Table 2.
Outcome of 15 Patients with Large and Giant Residual or Recurrent Vestibular Schwannomas Undergoing Total Resection
| Patient | CN VII Grade Preop* | Other Preop Neurological Deficits | CN VII Grade Postop* | Hydroce- phalus | Follow-Up | Complications |
|---|---|---|---|---|---|---|
| CN, cranial nerve. | ||||||
| 1 | II | Ataxia | IV | Yes | 10 years | No |
| 2 | VI | Ataxia, CN V, dysphasia | VI | Yes | 9 years | CSF leak/meningitis |
| 3 | I | No | III | No | 9 years | CSF leak |
| 4 | IV | Ataxia | VI | No | 8 years | No |
| 5 | VI | Ataxia, CN V, dysphasia | VI | Yes | 8 years | Facial numbness |
| 6 | VI | Ataxia, CN V | VI | Yes | 8 years | No |
| 7 | I | No | I | Yes | 5 years | No |
| 8 | VI | Ataxia | VI | Yes | 4 years | CN IX, X (transient) |
| 9 | III | No | IV | No | 3 years | No |
| 10 | VI | Ataxia, CN V, dysphasia, tracheotomy, gastrostomy | VI | Yes | 3 years | Facial numbness |
| 11 | VI | Ataxia, CN V | VI | Yes | 3 years | CSF leak |
| 12 | VI | Ataxia, dysphasia | VI | Yes | 2 years | No |
| 13 | VI | Ataxia, dysphasia, CN V | VI | Yes | 1.5 years | CN IX, X (transient), facial numbness |
| 14 | III | No | III | Yes | 4 months | No |
| 15 | I | No | III | Yes | 4 months | No |
Grade according to House and Brackmann.21
When the IAC had not been drilled during the previous surgery, it was easier to dissect the facial nerve within the IAC. Cranial nerve VII was identified at the brainstem in six cases. Intraoperative facial nerve monitoring helped in patients with a functioning cranial nerve VII. The facial nerve was preserved in six of seven patients with preoperative facial nerve function. In the remaining eight cases with complete preoperative facial paralysis, hypoglossal-facial anastomosis was performed 2 to 3 weeks after surgery in seven patients. Good functional results were obtained in these patients. In one patient with complete paralysis of the caudal cranial nerves, the face was rehabilitated with plastic surgery techniques.
Postoperatively, three patients complained of increased facial numbness. Transient worsening of bulbar cranial nerves palsy was observed in two cases. Three patients developed cerebrospinal fluid leak and were treated with lumbar drainage; one of these patients developed meningitis. The major complications in this series were meningitis in one patient and transient palsy of the caudal cranial nerves in two, one of which required transient tracheotomy and gastrostomy. These patients recovered well and developed no permanent morbidity. Tracheotomy and gastrostomy tubes previously placed in one patient after two surgical procedures and conformational radiotherapy were removed 3 months after the definitive tumor resection. No patients died. Histologically, all tumors were benign.
ILLUSTRATIVE CASES
Case 1
This 27-year-old man underwent two previous surgeries in another hospital 2 years before admission for removal of a large VS on the left side. The retrosigmoid approach had been used and only subtotal removal had been achieved. After the second procedure, the patient developed facial paralysis, swallowing disturbance, ataxia, meningitis, and hydrocephalus. After treatment of the meningitis and hydrocephalus with external ventricular drainage, a peritoneal shunt was placed.
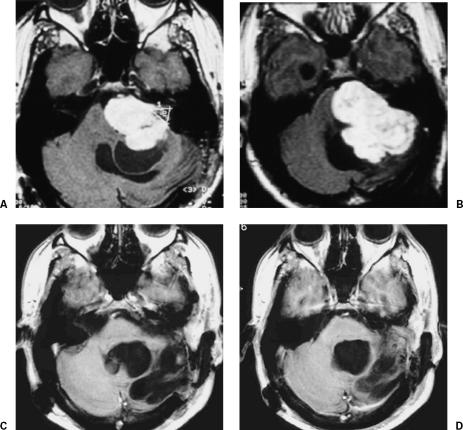
On admission to our clinic, the patient exhibited left-sided deafness, complete facial nerve paralysis, paralysis of the cranial nerves IX and X, and bulging in the left retromastoid region. MRI revealed a giant cystic residual or recurrent VS (Fig. 1).
Figure 1.
(A,B) Preoperative MRIs with gadolinium show a left giant cystic residual or recurrent VS compressing the brainstem and reaching the prepontine region. (C,D) Postoperative MRI with gadolinium 9 years after total removal. MRIs, magnetic resonance images; VS, vestibular schwannoma.
The tumor was totally removed through the retrosigmoid-transmeatal approach, and the patient developed no additional deficits. Hypoglossal/facial anastomosis was performed 2 weeks after tumor removal. Six months after surgery, he was able to return to work. Nine years after surgery, MRI showed no recurrence of the lesion (Fig. 1).
Case 2
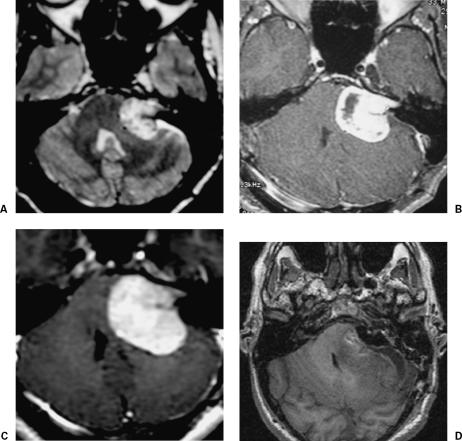
In February 2005, this 42-year-old man was admitted to our clinic with a history of one previous surgery elsewhere for partial removal of a 3.5-cm VS on the left 2.5 years earlier (Fig. 2A). The residual tumor was treated with gamma knife radiosurgery (Fig. 2B). Nonetheless, the remnant tumor showed progressive growth (Fig. 2C). At admission to our clinic, the patient presented with left-sided deafness and hydrocephalus. Initially, a shunt was placed, and the tumor was completely resected through a retrosigmoid/transmeatal approach (Fig. 2D). Although the IAC had not been drilled during the first surgery, dissection of the facial nerve at the brainstem was difficult due to scar tissue from the first surgery and radiosurgery. Postoperatively, the patient had no complications and House grade III facial nerve function.
Figure 2.
(A) Preoperative MRI with gadolinium show a left residual or recurrent VS compressing the brainstem. (B) MRI with gadolinium after gamma knife surgery. (C) MRI 2.5 years after surgery and radiosurgery shows marked growth of residual tumor. (D) Postoperative MRI confirms total tumor resection. MRI, magnetic resonance image; VS, vestibular schwannoma.
Case 3
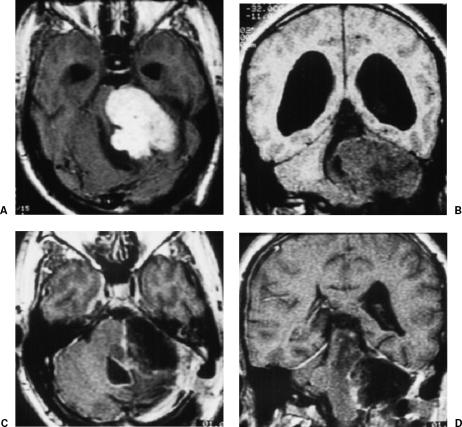
This 29-year-old man had been operated three times before for a giant left VS. Only partial resection of the tumor was achieved in all three surgical procedures. On follow-up MRI, the tumor showed further growth and the residual lesion received no treatment. At admission to our clinic, the patient's neurological examination showed paralysis of the left cranial nerves VIII and VII and ataxia. MRI showed a giant cystic recurrent or residual VS (Fig. 3). The tumor was totally resected through the retrosigmoid/transmeatal approach. The patient's postoperative course was uneventful. A hypoglossal/facial anastomosis was performed, and MRI confirmed total removal of the lesion (Fig. 3). The patient returned to his previous function as a pharmacist 6 months after surgery.
Figure 3.
(A) Axial and (B) coronal preoperative MRIs show a left giant cystic recurrent or residual VS and hydrocephalus after three previous surgeries. (C) Axial and (D) sagittal postoperative MRIs with gadolinium confirm total removal of the lesion. MRIs, magnetic resonance images; VS, vestibular schwannoma.
DISCUSSION
The management of VS has evolved significantly with the development of microsurgical techniques, the advent of new radiological diagnostic procedures, and neurophysiological intraoperative monitoring. Many tumors are diagnosed when they are small or of medium size. In these cases, the goals of surgery are preservation of facial nerve function and hearing. For larger lesions compressing the brainstem, radical removal with preservation of facial nerve function without additional morbidity or mortality are the goals of surgical resection.
Most small or medium-sized tumors are removed completely. Larger and giant tumors, especially cystic VS, may be intentionally or unintentionally subtotally resected for various reasons,6,7 for example, a new presentation of the tumor after its complete removal or residual lesion.8 The growth rate of residual tumor is unpredictable,1,9,10,11 and some tumor remnants may not grow. In our series, all patients had residual tumors that had grown on follow-up imaging. We prefer to classify these lesions as residual or recurrent VS.
When excision is incomplete, the recurrence rate is usually high. Sasaki and associates12 reported recurrence rates of 29% (5 of 17 patients) and 25% (2 of 8 patients) for near-total and subtotal resection and no recurrences after total resection. Bloch and colleagues13 reported 52 patients with subtotal removal, 33 with near-total resections (remnant ≤ 25 mm and ≤ 2 mm thick), and 19 with subtotal resections (any larger remnant). Recurrences occurred in 1 of 33 patients (3%) who had a near-total resection and in 6 of 19 patients (32%) who had a subtotal resection. All recurrences were observed in the midcerebellopontine angle after the translabyrinthine approach. Most authors reported a very low recurrence rate after complete tumor excision7,14,15,16; however, a higher recurrence rate after total removal has also been observed.17
Surgical resection of residual VS is usually difficult due to scar tissue and the absence of a clear arachnoid plane between tumor and brainstem, vessels, and nerves. Removal of large and giant residual or recurrent VS is more difficult, even for the most experienced surgeon. The few available reports on the treatment and outcome of residual VS are in otolaryngological journals and in series of patients previously operated through the retrosigmoid approach in neurosurgical departments.1,3,4 Recurrent tumors are usually treated when they are still small because these patients are carefully followed with postoperative CT or MRI. Our patients (mean age, 37.8 years) with large and giant residual/recurrent VS were young and followed with MRI. They received no treatment, despite proven growth of residual tumor. Most of these patients had complications after their previous surgeries. Both patients and their original surgeons were afraid that a new surgical procedure or even treatment with radiotherapy could cause further complications.
Samii and Matthies7 published the largest series on VS operated by one surgeon. They achieved excellent results with only 11 deaths (1.1%) in the entire series. In this series, 62 patients had residual or recurrent tumors, 56 of whom had undergone surgery in other hospitals (24 subtotal removals and 32 biopsies). Size of lesion and outcomes were not presented. Two patients died after surgery due to recurrent tumors. In other reported series of 179 cases, 11 patients (6%) had undergone previous surgery.14 Again, tumor size and outcome of these cases were not described.
Sanna and colleagues4 presented a series of 23 residual VS. Four patients had large lesions (4.0 cm) and 1 had a giant VS (5.0 cm). Two patients had preoperative facial nerve paralysis. Radical removal was possible in 4 of these patients, but the facial nerve could not be preserved in any case. The transcochlear approach was used in 3 patients and the translabyrinthine approach in 2.
Most articles on recurrent or residual VS have focused on small or middle-sized recurrent tumors. No single article focusing on the surgical treatment and outcome of giant residual or recurrent tumors could be found in the literature. In our series, all 15 patients had large or giant tumors compressing the brainstem. Nine patients underwent at least two previous surgical procedures. All lesions were totally removed through a retrosigmoid-transmeatal approach. There were no deaths. Facial nerve function was preserved in 6 of the 7 patients with preoperative functional facial nerve. In our opinion, cranial nerve XII to VII anastomosis should be avoided in patients with complete caudal cranial nerves paralysis (1 case in this series). In such cases the face can be rehabilitated through plastic surgery procedures, cross-face anastomosis, or both.
Unger et al18 reported good outcomes in 50 patients treated with gamma knife radiosurgery after previous subtotal microsurgical resection or after a recurrence after total resection of VS. The median treatment volume was 3.4 cc3 with a median dose to the tumor margin of 13 Gy. The median follow-up was 75 months (range, 42 to 114 months), and the control rate was 96%. The three patients in this series treated after initial surgical removal with radiosurgery or conformational radiotherapy showed growth of residual tumor. Radiosurgery is not recommended for large lesions associated with significant compression of the brainstem. These tumors usually require surgical resection.19 Radiosurgical doses and tumor dimensions were considered the two most important risk factors for injuries involving cranial nerves VII and V.20
CONCLUSION
The growth rate of residual VS is unpredictable. Surgery of VS should be performed only in centers where a high rate of total removal with low morbidity can be achieved. Large and giant residual or recurrent tumors can be totally resected through the retrosigmoid/transmeatal approach with low morbidity and mortality rates. In these cases, scar tissue and the absence of an arachnoidal plane make preservation of facial nerve difficult, even for the most experienced surgeon.
REFERENCES
- Beatty C W, Ebersold M J, Harner S G. Residual and recurrent acoustic neuromas. Laryngoscope. 1987;97:1168–1171. doi: 10.1288/00005537-198710000-00009. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59:283–291. doi: 10.1016/s0090-3019(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Roberson J B, Jr, Brackmann D E, Hitselberger W E. Acoustic neuroma recurrence after suboccipital resection: management with translabyrinthine resection. Am J Otol. 1996;17:307–311. [PubMed] [Google Scholar]
- Sanna M, Falcioni M, Taibah A, De Donato G, Russo A, Piccirillo E. Treatment of residual schwannoma. Otol Neurotol. 2002;23:980–987. doi: 10.1097/00129492-200211000-00028. [DOI] [PubMed] [Google Scholar]
- Shea J J, 3rd, Hitselberger W E, Benecke J E, Jr, Brackmann D E. Recurrence rate of partially resected acoustic tumors. Am J Otol. 1985;(Suppl):107–109. [PubMed] [Google Scholar]
- Matthies C, Samii M. Vestibular schwannomas and auditory function: options in large T3 and T4 tumors? Neurochirurgie. 2002;48:461–470. [PubMed] [Google Scholar]
- Samii M, Matthies C. Management of 1000 vestibular schwannomas (acoustic neuromas): surgical management and results with an emphasis on complications and how to avoid them. Neurosurgery. 1997;40:11–23. doi: 10.1097/00006123-199701000-00002. [DOI] [PubMed] [Google Scholar]
- Shelton C. Unilateral acoustic tumors: how often do they recur after translabyrinthine removal? Laryngoscope. 1995;105:958–966. doi: 10.1288/00005537-199509000-00016. [DOI] [PubMed] [Google Scholar]
- El-Kashlan H K, Zeitoun H, Arts H A, Hoff J T, Telian S A. Recurrence of acoustic neuroma after incomplete resection. Am J Otol. 2000;21:389–392. doi: 10.1016/s0196-0709(00)80049-6. [DOI] [PubMed] [Google Scholar]
- Gamache F, Patterson R. In: Tos M, Thomsen J, editor. Acoustic Neuroma. Amsterdam, The Netherlands: Kugler; 1992. Growth rates for residual and recurrent acoustic neuroma. pp. 705–707.
- Pace-Balzan A, Lye R H, Ramsden R T, Chandler C, Gillespie J E, Dutton J M. In: Tos M, Thomsen J, editor. Acoustic Neuroma. Amsterdam, The Netherlands: Kugler; 1992. Growth characteristics of acoustic neuromas with particular reference to the fate of capsule fragments remaining after tumor removal: implications for patient management. pp. 701–703.
- Sakaki S, Nakagawa K, Hatakeyama T, Murakami Y, Ohue S, Matsuoka K. Recurrence after incompletely resected acousticus neurinomas. Med J Osaka Univ. 1991;40(1–4):59–66. [PubMed] [Google Scholar]
- Bloch D C, Oghalai J S, Jackler R K, Osofsky M, Pitts L H. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg. 2004;130:104–112. doi: 10.1016/S0194-5998(03)01598-5. [DOI] [PubMed] [Google Scholar]
- Gormley W B, Sekhar L N, Wright D C, Kamerer D, Schessel D. Acoustic neuromas: results of current surgical management. Neurosurgery. 1997;41:50–60. doi: 10.1097/00006123-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Lanman T H, Brackmann D E, Hitselberger W E, Subin B. Report of 190 consecutive cases of large acoustic tumors (vestibular schwannoma) removed via the translabyrinthine approach. J Neurosurg. 1999;90:617–623. doi: 10.3171/jns.1999.90.4.0617. [DOI] [PubMed] [Google Scholar]
- Ramina R, Maniglia J J, Meneses M S, et al. Acoustic neurinomas. Diagnosis and treatment. Arq Neuropsiquiatr. 1997;55(3A):393–402. doi: 10.1590/s0004-282x1997000300007. [DOI] [PubMed] [Google Scholar]
- Thomassin J M, Pellet W, Epron J P, Braccini F, Roche P H. Recurrent acoustic neurinoma after complete surgical resection [in French] Ann Otolaryngol Chir Cervicofac. 2001;118:3–10. [PubMed] [Google Scholar]
- Unger F, Walch C, Papaefthymiou G, Feichtinger K, Trummer M, Pendl G. Radiosurgery of residual and recurrent vestibular schwannomas. Acta Neurochir (Wien) 2002;144:671–677. doi: 10.1007/s00701-002-0950-5. [DOI] [PubMed] [Google Scholar]
- Kondziolka D, Lunsford L D, Flickinger J C. Acoustic tumors: operation versus radiation—making sense of opposing viewpoints. Part II Acoustic neuromas: sorting out management options. Clin Neurosurg. 2003;50:313–328. [PubMed] [Google Scholar]
- Ito K, Shin M, Matsuzaki M, Sugasawa K, Sasaki T. Risk factors for neurological complications after acoustic neurinoma radiosurgery: refinement from further experiences. Int J Radiat Oncol Biol Phys. 2000;48:75–80. doi: 10.1016/s0360-3016(00)00570-8. [DOI] [PubMed] [Google Scholar]
- House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]