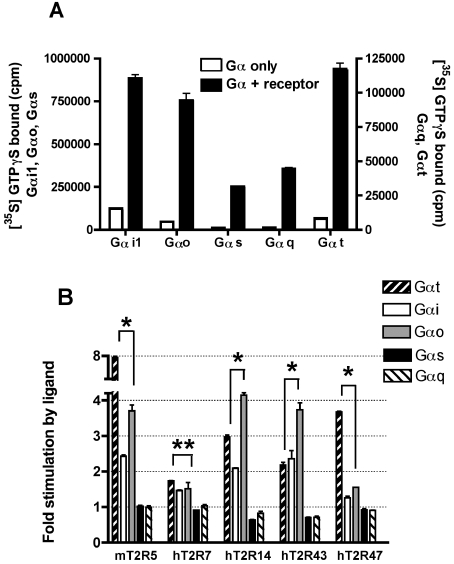
Figure 5. G-protein selectivity of T2Rs among members of the Gαi family.
(A) The activity of each purified Gα subunit was confirmed using either Sepia rhodopsin or, in the case of Gαs, Sf9-expressed β2-adrenergic receptor in the presence of 1 μM isoproterenol. Robust receptor-catalysed GDP/GTP exchange was observed for each Gα subunit tested. (B) Urea-washed membranes from mT2R5-, hT2R7-, hT2R14-, hT2R43- and hT2R47-expressing Sf9 cells containing approximately 8 pmol of rhodopsin epitope were incubated with 200 nM of the indicated Gα subunit and 1 μM Gβ1γ1 in the absence or presence of activating ligand (300 μM aristolochic acid for hT2R14 and hT2R43, 30 μM cycloheximide for mT2R5, 300 μM denatonium for hT2R47 and 300 μM strychnine for hT2R7). Receptor activation of Gαi1, Gαo and Gαs was measured using a competitive GDP reaction mixture for 4 min at 30 °C, while receptor activation for transducin (Gαt) and Gαq was measured with 1 μM GTP[S] (GTPγS) for 20 min at 30 °C. Data presented are fold-increases in GTP[S] binding comparing reactions which included ligand and those without ligand. *P<0.005; **P=0.2 (unpaired t test).