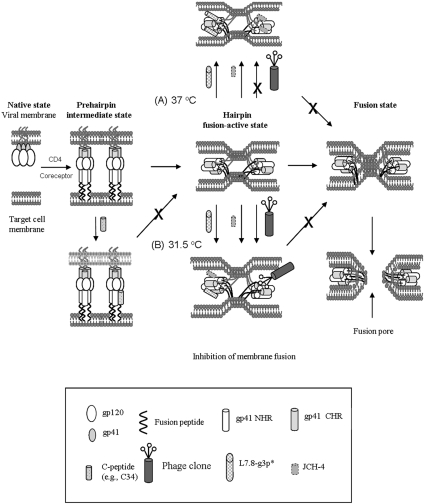
Figure 7. Model showing interaction of the HXXNPF motif-containing molecules with the 6-HB and inhibition of HIV-1 Env-mediated membrane fusion.
After viral gp120 binds to CD4 and a co-receptor on the target cell, gp41 changes its conformation. The fusion peptide inserts into the target cell membrane and the NHR and CHR regions interact with each other to form a 6-HB, bringing the viral and the cellular membranes into close proximity. The gp41 core may play an important role in the late stage of membrane-fusion events, e.g. formation of the fusion pores. Binding of the small molecules L7.8-g3p* and the peptide JCH-4 to the N-helices in the 6-HB at the fusion-active state may prevent the further fusion between the viral and target cell membranes (A and B). The larger molecules, e.g. the phage clone L7.8 and mAb NC-1, though they can bind to the 6-HB formed by the N- and C-peptides in vitro, they cannot access the fusion-active gp41 core formed by the viral gp41 NHR and CHR regions at the conventional temperature (37 °C), resulting in their inability to inhibit HIV fusion (A). However, when suboptimal temperature (31.5 °C) is used to slow down the fusion process and prolong the exposure time of the gp41 coiled-coil structure, the phage clone L7.8 may be able to gain access to the gp41 core and inhibit membrane fusion (B).