Fig. 2.
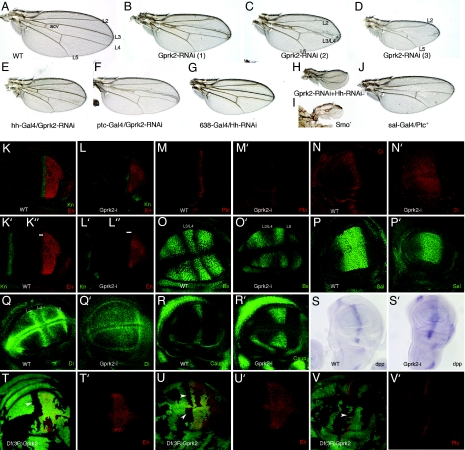
Gprk2 is required for Hh signaling. (A) Wild-type wing showing the position of the longitudinal veins L2 to L5. acv, anterior cross vein. (B–D) Adult wings resulting from ectopic expression of interference Gprk2 RNA in Gal4–638/UAS-Gprk2i flies. Mild phenotypes consist of a moderate reduction of wing size and the shortening of the distance between the L3 and L4 veins [B; Gprk2-RNAi (1)]. Medium phenotypes are characterized by partial fusion of the L3 and L4 veins [C; Gprk2-RNAi (2)]. Strong phenotypes consist in greater reductions of wing size and the disappearance of the L3 and L4 veins [D; Gprk2-RNAi (3)]. (E) Adult wing from Gal4-hh/UAS-Gprk2i flies, expressing interference Gprk2 RNA in the posterior compartment. (F) Adult wing from Gal4-ptc/UAS-Gprk2i flies, expressing interference Gprk2 RNA in anterior cells close to the A/P compartment boundary (compare to B and C). (G) Hh loss-of-function phenotype in 638-Gal4/UAS-hh-RNAi flies consists of wing size reduction, shortening of the distance between the veins L3 and L4, and loss of the anterior cross vein (acv in A). (H) Severe hh loss-of-function phenotype in 638-Gal4/UAS-hh-RNAi; UAS-Gprk2i/+; compare to the expression of only hh-RNAi (G) or only Gprk2-RNAi (B–D). (I) Severe hh loss-of function phenotype in Gal4–638/+; FRT42 smo2/FRT42 M (2)l2; UAS-FLP/+. These wings, formed by homozygous smo2 cells, are extremely reduced in size and have lost most pattern elements. (J) Hh loss-of-function phenotype caused by ectopic expression of ptc (sal-Gal4/UAS-ptc) is very similar to loss of Gprk2 function (compare to D). (K–K″) Expression of Knot (Kn; K and K′, green) and Engrailed (En; K and K″, red) in a third instar wild-type wing imaginal disc. (L–L″) Loss of Gprk2 reduces Knot expression (Kn; L and L′, green) and eliminates anterior expression of Engrailed (En; L, L″, red). The white lines in K″ and L″ delimit the anterior domain of En expression. (M and M′) Expression of Ptc in wild-type discs (wt; M) and in Gal4–638/UAS-Gprk2i (Gprk2-i; M′). Loss of Gprk2 reduces the expression of Ptc. (N and N′) Expression of Ci in wild-type discs (wt; N) and in Gal4–638/UAS-Gprk2i wing discs (Gprk2-i; N′). (O and O′) Expression of Bs in wild-type discs (wt; O) and in Gal4–638/UAS-Gprk2i wing discs (Gprk2-i; O′). Loss of Gprk2 eliminates the L3/L4 intervein domain of Bs expression. (P and P′) Expression of the Dpp target gene Sal in wild-type (wt; P) and Gal4–638/UAS-Gprk2i discs (Gprk2-i; P′). Overall, the level and anterior-posterior extent of the Sal domain is not affected. (Q and Q′) Expression of Dl in wild-type discs (wt; Q) and in Gal4–638/UAS-Gprk2i (Gprk2-i; Q′). Loss of Gprk2 reduces Dl expression in the L3 and L4 veins. (R and R′) Wing discs showing the expression of Caupolican (Caup) in wild-type (wt; R) and Gal4–638/UAS-Gprk2i wing discs (Gprk2-i; R′). Loss of Gprk2 expands Caup. (S and S′) In situ hybridization with a dpp RNA probe in wild-type (wt; S) and Gal4–638/UAS-Gprk2i (Gprk2-i; S′) wing discs. Upon a reduction in Gprk2 levels, dpp expression occurs at lower levels and in an expanded domain. (T–V) Clones of Df(3R)Gprk2 cells induced in hs-FLP1.22; FRT82 Df(3R)Gprk2/ FRP82 M (3)w Ubi-GFP discs at 48–72 h after egg laying. Homozygous Gprk2− cells are labeled by the absence of GFP and appear as black spots. In T and T′ and U and U′, the expression of En (En; red) is shown, and in V and V′, the expression of Ptc is shown in red. In anterior Gprk2 mutant cells located close to the A/P compartment boundary, the expression of En (T–U′) and Ptc (V and V′) is not detected. T′, U′, and V′ show the red channels of T, U, and V, respectively.