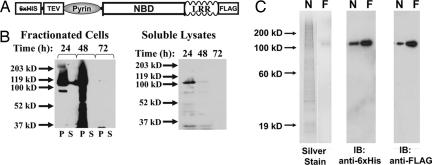
Fig. 1.
Recombinant cryopyrin expressed in insect cell culture can be purified to homogeneity by affinity chromatography. (A) Schematic of the recombinant tags on dual-tagged cryopyrin. N-terminal hexahistidine tag and TEV protease site and C-terminal FLAG tag are indicated. The Pyrin domain, NBD, and LRR domains indicate domains of the native protein. (B) Dual-tagged cryopyrin was expressed in Hi5 Insect cells by infection with recombinant baculovirus (multiplicity of infection = 0.5). After the indicated times, the cells were harvested, flash-frozen, resuspended in lysis buffer, and sonicated. After centrifugation at 15,000 × g for 1 h, the supernatants (S, soluble fraction) were removed and the pellets (P, insoluble fraction) were resuspended in an equal volume of 1× SDS/PAGE loading buffer. The soluble (S, supernatant) and insoluble (P, pellet) fractions (Left) were subjected to immunoblot analysis with anti-FLAG antibody. The soluble lysates from the infected cells fractions are run alone (Right) and demonstrate that immunoreactive material in the second lane in Left is not bleed-over from a neighboring lane. (C) Recombinant cryopyrin was purified by sequential affinity chromatography. Protein eluted from the nickel (N, 200 ng) followed by the anti-FLAG (F, 50 ng) columns were subjected to silver staining (Left) or immunoblot with indicated antibodies (Center and Right).