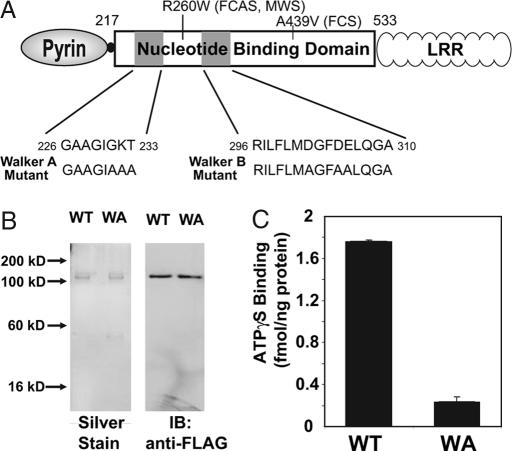
Fig. 3.
Mutation of the Walker A motif of cryopyrin abrogates its ATP-binding activity. (A) WT and Walker A mutant variants of cryopyrin. Mutated Walker A sequence is shown. Walker B mutation, used in SI Table 1, is noted. Disease-associated mutations, R260W and A439V, used in Fig. 5, are also noted. (B) WT and Walker A mutant (WA) cryopyrin were purified by sequential affinity chromatography as described in Fig. 1A. Silver staining (Left) and immunoblot analysis (Right) of 50 ng of each of the purified proteins is shown. (C) ATPγ S binding activity of the indicated proteins was assayed at 60 min in the presence of 200 nM ATPγ S. Error bars represent standard deviation of ATP-binding measurements determined in triplicate.