Abstract
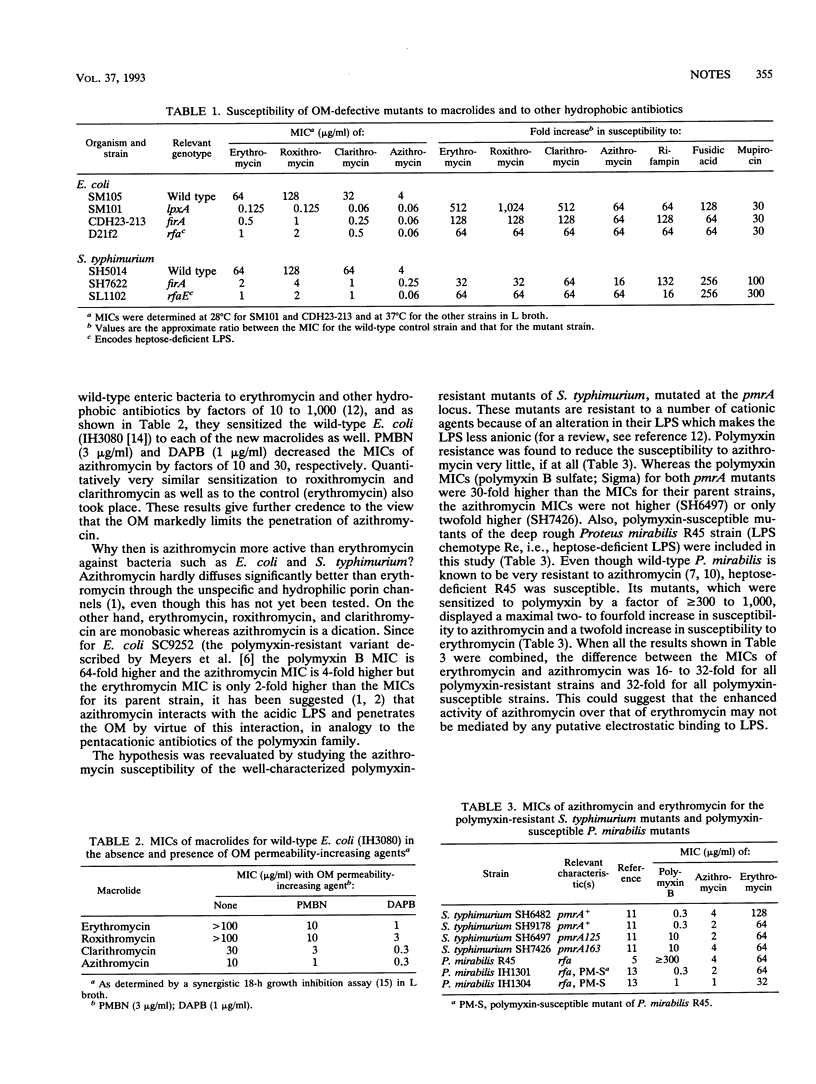
Mutations which severely affect the function of the outer membrane of Escherichia coli and Salmonella typhimurium (lpxA and firA mutations of lipid A synthesis and rfaE mutation of the lipopolysaccharide inner-core synthesis) were found to decrease the MICs of erythromycin, roxithromycin, clarithromycin, and azithromycin by factors of 32 to 512, 32 to 1,024, 64 to 512, and 16 to 64, respectively. The sensitization factors for three other hydrophobic antibiotics (rifampin, fusidic acid, and mupirocin) ranged from 16 to 300. The outer membrane permeability-increasing agents polymyxin B nonapeptide (3 micrograms/ml) and deacylpolymyxin B (1 microgram/ml) sensitized wild-type E. coli to azithromycin by factors of 10 and 30, respectively. Quantitatively very similar sensitization to the other macrolides took place. Polymyxin-resistant pmrA mutants of S. typhimurium displayed no cross-resistance to azithromycin. Proteus mirabilis mutants which were sensitized to polymyxin by a factor of > or = 300 to > or = 1,000 had a maximal two- to fourfold increase in sensitivity to azithromycin. These results indicate that azithromycin and the other new macrolides use the hydrophobic pathway across the outer membrane and that the intact outer membrane is an effective barrier against them. Furthermore, the results indicate that azithromycin, in contrast to polymyxin, does not effectively diffuse through the outer membrane by interacting electrostatically with the lipopolysaccharide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farmer S., Li Z. S., Hancock R. E. Influence of outer membrane mutations on susceptibility of Escherichia coli to the dibasic macrolide azithromycin. J Antimicrob Chemother. 1992 Jan;29(1):27–33. doi: 10.1093/jac/29.1.27. [DOI] [PubMed] [Google Scholar]
- Hirvas L., Koski P., Vaara M. Identification and sequence analysis of the gene mutated in the conditionally lethal outer membrane permeability mutant SS-C of Salmonella typhimurium. EMBO J. 1991 Apr;10(4):1017–1023. doi: 10.1002/j.1460-2075.1991.tb08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirst H. A., Sides G. D. New directions for macrolide antibiotics: structural modifications and in vitro activity. Antimicrob Agents Chemother. 1989 Sep;33(9):1413–1418. doi: 10.1128/aac.33.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelko K. Proteus mirabilis: taxonomic position, peculiarities of growth, components of the cell envelope. Curr Top Microbiol Immunol. 1986;129:181–215. doi: 10.1007/978-3-642-71399-6_3. [DOI] [PubMed] [Google Scholar]
- Meyers E., Parker W. L., Brown W. E., Linnett P., Strominger J. L. EM49: a new polypeptide antibiotic active against cell membranes. Ann N Y Acad Sci. 1974 May 10;235(0):493–501. doi: 10.1111/j.1749-6632.1974.tb43286.x. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Saha G., Labthavikul P. Comparative in vitro activity of the new oral macrolide azithromycin. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):541–544. doi: 10.1007/BF01962611. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992 Sep;56(3):395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. Increased outer membrane resistance to ethylenediaminetetraacetate and cations in novel lipid A mutants. J Bacteriol. 1981 Nov;148(2):426–434. doi: 10.1128/jb.148.2.426-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Viljanen P., Vaara T., Mäkelä P. H. An outer membrane-disorganizing peptide PMBN sensitizes E. coli strains to serum bactericidal action. J Immunol. 1984 May;132(5):2582–2589. [PubMed] [Google Scholar]
- Viljanen P., Matsunaga H., Kimura Y., Vaara M. The outer membrane permeability-increasing action of deacylpolymyxins. J Antibiot (Tokyo) 1991 May;44(5):517–523. doi: 10.7164/antibiotics.44.517. [DOI] [PubMed] [Google Scholar]
- Vuorio R., Vaara M. Mutants carrying conditionally lethal mutations in outer membrane genes omsA and firA (ssc) are phenotypically similar, and omsA is allelic to firA. J Bacteriol. 1992 Nov;174(22):7090–7097. doi: 10.1128/jb.174.22.7090-7097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorio R., Vaara M. The lipid A biosynthesis mutation lpxA2 of Escherichia coli results in drastic antibiotic supersusceptibility. Antimicrob Agents Chemother. 1992 Apr;36(4):826–829. doi: 10.1128/aac.36.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]


