Figure 6.
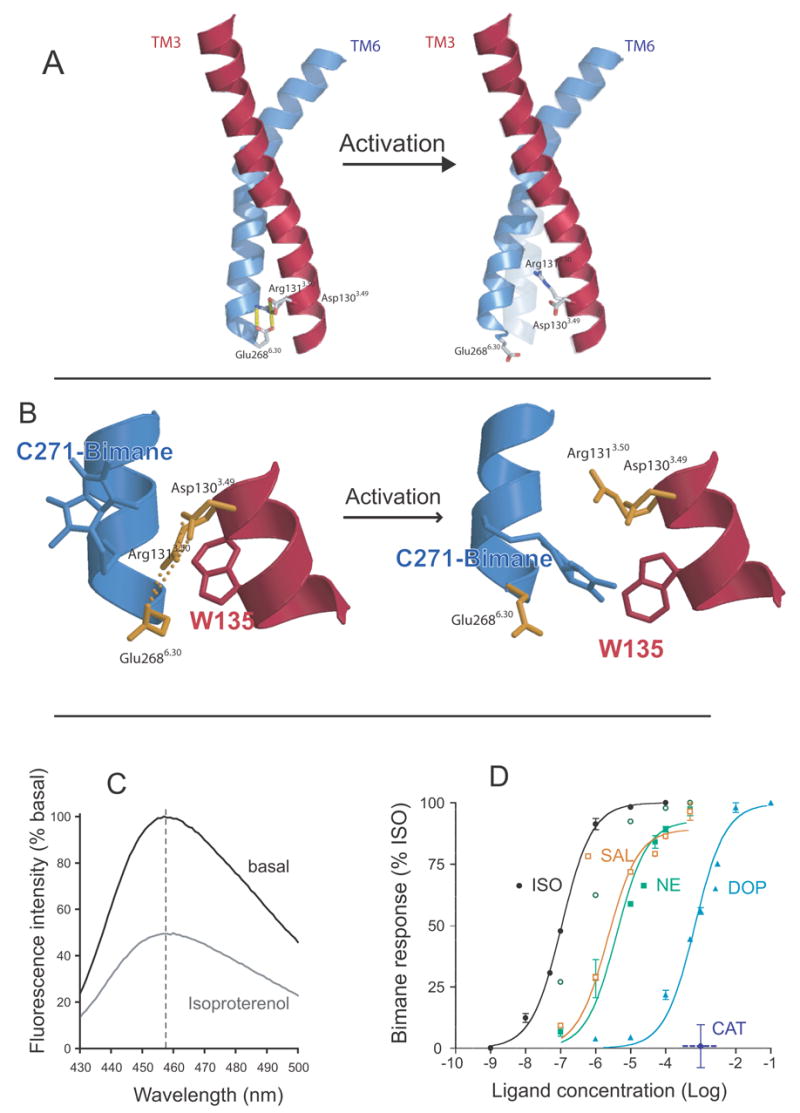
Fluorescence spectroscopy to monitor disruption of the ionic lock in the β2AR. A. Model of TM3 (red) and TM6 (blue) from the β2AR depicting the amino acids that comprise the ionic lock at the cytoplasmic end of these TM segments. B. Close up view of the ionic lock and the modifications made to monitor conformational changes in this region. Alanine 271 was mutated to cysteine (C271) and isoleucine 135 was mutated to tryptophan (W135). C271 was labeled with monobromobimane in purified β2AR. Upon activation, W135 moves closer to bimane on C271 and quenches fluorescence. C. Emission spectrum of bimane on C271 before and after activation by the agonist isoproterenol. D. Effect of different ligands on disruption of the ionic lock as determined by bimane fluorescence. The partial agonists dopamine and salbutamol are as effective at disrupting the ionic lock as the full agonists norepinephrine and isoproterenol. Only catechol has no effect on the ionic lock. These data are adapted from Yao et al. [71].
