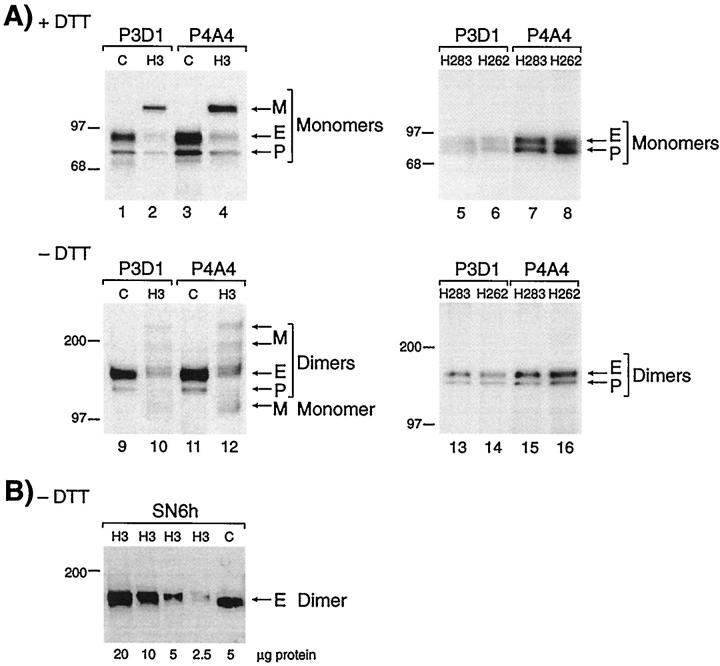
Figure 2.
Analysis of peripheral blood-activated monocytes shows reduced levels of normal endoglin and presence of a mutant protein in patient H3 but not in his parents. A: Cells from the clinically affected patient H3 (family 2), and a normal control (C) were labeled with 35S-methionine, solubilized in Triton X-100, and immunoprecipitated with mAb P3D1 or P4A4 to endoglin. Equivalent amounts of labeled proteins were fractionated on 4 to 12% gradient SDS-PAGE, under reducing (lanes 1–4) and nonreducing (lanes 9–12) conditions. Fully processed endoglin (E) and partially glycosylated precursor (P) were observed in patient H3 at reduced levels compared with control, and a mutant protein (M) is noted. Cells from individuals H262 and H283, mother and father of patient H3, were also analyzed in the same manner (lanes 5–8 and 13–16), normal E and P forms of endoglin were seen at normal levels, and no mutant protein was observed. B: Activated monocytes from patient H3 were lysed in Triton X-100, and various amounts of proteins were fractionated on 8% sodium dodecyl sulfate-polyacrylamide gels, under nonreducing conditions (without dithiothreitol). Gels were transferred and analyzed by Western blot analysis, using mAb SN6h to endoglin. The control (C) was an extract from HUVEC. Only the normal dimers of endoglin (E) are detected by Western blot analysis. Molecular mass markers in kilodaltons are indicated.