Abstract
Mitotic figures of fibroblasts are seen within invasive ductal carcinoma (IDC) of the breast. This suggests that the proliferative activity of fibroblasts may play an important role in IDC tumor progression. The purpose of this study was to examine whether the proliferative activity of fibroblasts can predict lymph node metastasis (LNM) or distant-organ metastasis (DOM) of IDCs. Two hundred four consecutive patients with IDC of the breast surgically treated at the National Cancer Center Hospital East constituted the basis of this study. Proliferative activity of fibroblasts was immunohistochemically evaluated by the mouse MIB-1 monoclonal antibody against Ki-67 antigen. The MIB-1 labeling index was the percentage of fibroblasts with positively stained nuclei, and fields for cell counting were selected in inner and outer areas within IDCs. In both areas, 300 fibroblasts were counted in each high-power field. The significance of proliferative activity of fibroblasts on LNM or DOM was compared with well-known prognostic parameters. Multivariate analyses demonstrated that a MIB-1 labeling index of more than 10% of fibroblasts in the inner area of IDCs significantly increased the relative risk of LNM and hazard rate of DOM (P < 0.001 and P = 0.007, respectively). The present study indicated that the metastatic ability of IDCs is closely dependent on proliferative activity of fibroblasts in the inner area.
The proliferative activity of a tumor can be assessed by DNA flow cytometric determination of S-phase fraction, thymidine-labeling index by autoradiographic method, or immunohistochemistry with MIB-1 monoclonal antibody generated against the Ki-67 antigen. 1-4 The MIB-1 labeling index (LI) is a most convenient method for evaluating proliferative activity of tumor in routine practice.
It has been reported that the proliferative activity of tumor cells plays an important role in tumor progression of breast cancer, and influences breast cancer patient outcome. 5,6 In routine examination, mitotic figures of stromal fibroblasts as well as those of the tumor cells are seen in some areas within the tumor. This suggests that the proliferative activity of fibroblasts may play an important role in tumor progression of breast cancer. Tumor-stromal interaction plays an important role in tumor invasion or metastasis. 7,8 However, to date no study has evaluated the role of proliferative activity of fibroblasts in tumor progression.
The purpose of this study was to examine whether the proliferative activity of fibroblasts plays an important role on tumor progression of invasive ductal carcinoma (IDC) of the breast. The significance of proliferative activity of fibroblasts on lymph node metastasis (LNM) or distant-organ metastasis (DOM) of IDC patients was compared with well-known histological parameters, proliferative activity of tumor cells, c-erbB-2 protein expression, or tumor angiogenesis, which are significant prognostic factors for breast cancer patients. 5,6,9-11 The present study demonstrated that proliferative activity of fibroblasts is an independent parameter predicting LNM or DOM of IDCs in multivariate analyses, and showed that metastatic ability of IDCs is closely dependent on proliferative activity of fibroblasts in the inner area.
Materials and Methods
Cases
Two hundred four consecutive patients with IDC of the breast surgically treated between July 1992 and November 1996 at the National Cancer Center Hospital East, constituted the basis of this study. Clinical information was obtained from the patients’ medical records. All of the patients were Japanese women ranging in age from 28 to 87 years (average, 53 years), and all had a solitary lesion. One hundred five patients were premenopausal and 101 postmenopausal. Standard radical mastectomy was performed in 15 patients, modified radical mastectomy in 186, and quadrantectomy in five. Axillary lymph node dissection was carried out in 196 patients. None of the patients received radiotherapy or chemotherapy before surgery. After operation, 149 patients were treated with adjuvant therapy (chemotherapy or hormone therapy). Only three patients had received postoperative radiation therapy. All tumors were classified according to the pathological TNM (pTNM) classification. 12
For pathological examination, the surgically resected specimens were fixed in 10% formalin overnight at 4C and the entire tumor was cut into slices at intervals of 0.5 to 0.7 cm. The size and gross appearances of the tumor were recorded, and the former was validated by comparison with tumor size on histological slides. Multiple histological sections were taken from each tumor to measure the maximum tumor diameter and area. The sections were processed routinely and embedded in paraffin.
Histological Examination
Serial sections of each tumor were cut from the paraffin blocks. One section was stained with hematoxylin and eosin (H&E) and examined pathologically to confirm the diagnosis. The remaining sections were used for immunohistochemistry. All tumors were classified according to the guidelines of the World Health Organization, 13 and their histological grade was evaluated by the classification of Elston and Ellis. 14
Immunohistochemistry
Immunohistochemical staining was performed by the avidin-biotin-peroxidase complex technique. 15 The primary antibodies used were an affinity-purified monoclonal antibody against estrogen receptor (ER) protein (1D5; DAKO, Glrostrup, Denmark) used at a 1:100 dilution, polyclonal antibody-specific for the 185-kd c-erbB-2 protein (Nichirei, Tokyo, Japan) used at a 1:200 dilution, a mouse monoclonal antibody against Ki-67 antigen (MIB-1, Immunotech, Marseille, France), applied at a 1:50 dilution, and a mouse monoclonal antibody against human endothelial cells (CD31, JC/70A; DAKO), used at a 1:50 dilution. Microwave treatment was performed before immunohistochemical staining for MIB-1. 16 Sections were treated with 0.05% pepsin before the staining of CD31. After immunostaining, the sections were counter-stained with H&E. Sections of IDC positive for ER protein, c-erbB-2 protein, MIB-1, and CD31 were used each time as a positive control. As a negative control, the primary antibody was replaced with normal rabbit serum or normal mouse immunoglobulin.
Assessment of Immunohistochemical Results
Tumor cell nuclei that stained brown to dark brown were considered positive for ER or MIB-1, and faintly stained nuclei were considered negative. Nuclear staining for ER was only considered positive when more than 10% of the tumor cells in the entire tumor area were judged to be positive. Tumors were judged to be positive for c-erbB-2 protein when the cell membrane of more than 10% of the tumor cells throughout the tumor stained positive.
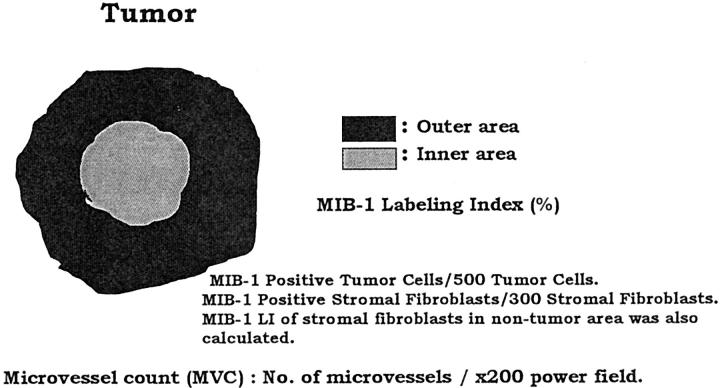
The MIB-1 LI is the percentage of tumor cells or fibroblasts with positively stained nuclei among the total number of tumor cells or fibroblasts counted. The fields for cell counting were selected in the inner and outer areas within the tumor (Figure 1) ▶ . The inner area was defined as a circle, the radius of which was half of the radius of the tumor. The outer area was the zone surrounding the inner area of the tumor. In inner and outer areas, at least 500 tumor cells were counted in each high-power field (×400), and 1,000 tumor cells were counted in one IDC. A total of 300 fibroblasts in the inner and outer areas were counted in each high-power field, and 600 fibroblasts within the tumor were counted in one IDC. In addition, 300 fibroblasts in a nontumor area, which was more than 5 mm from the tumor, were also counted in all IDCs.
Figure 1.
Schematic drawing of assessments of MIB-1 LI of tumor cells or fibroblasts, and of MVC of IDCs.
Microvessel count (MVC) was assessed in the inner and outer areas of the tumor (Figure 1) ▶ . In each tumor area, the field of highest neovascularity was identified by scanning the tumor sections stained for CD31 at low power magnification (×40 and ×100 total magnification). After the fields of highest neovascularity were identified, the individual microvessels were counted within ×200 fields (×20 objective and ×10 ocular, 0.384 mm 2 per fields). Any brown-staining endothelial cell clusters consisting of two or more cells with formation of a definite lumen clearly separate from the adjacent microvessels, tumor cells, and other connective tissue elements, were considered to be a single, countable microvessel. Lumina without a rim of CD31-positive cells were not counted, even if they contained blood cells. Results were expressed as the highest number of microvessels in any single ×200 field. 17 Two of the authors (TH and AO) investigated MIB-1 LI of tumor cells or fibroblasts, and MVC. If MIB-1 LI or MVC counted between TH and AO was almost equal, the MIB-1 LI or MVC counted by TH was used in this study. However, if there was a discrepancy in MIB-LI or MVC, we re-examined them to gain the consensus of each other.
Outcome
The survival of the patients was determined by follow-up to June of 1999, over a median period of 41 months. One hundred fifty-nine patients were alive and well, 47 had tumor recurrence, 29 had DOM, and 22 had died of their disease. Measurement of DOM-free survival, and overall survival (OS) was started at the time of surgery. Tumor relapse was defined as any evidence of metastasis or local recurrence. DOM was observed in the following organs: 1) bone, 12 cases; 2) liver, nine cases; 3) lung, six cases; and 4) brain, two cases. Only deaths due to breast cancer were considered for the purpose of this study, and all cases that died of the disease had DOM.
Statistical Analysis
Correlation between proliferative activity of tumor cells and fibroblasts in the inner and outer areas of a tumor was analyzed by linear correlation. In addition, correlation between proliferative activities of tumor cells or fibroblasts and number of mitotic figures of tumor cells was also assessed.
We attempted to examine by the t-test whether there is a difference in proliferative activity of tumor cells or fibroblasts in the inner and outer areas in tumors with ≤20 mm, 21 to 50 mm, and >50 mm in size, respectively. 12
In all cases, correlations between proliferative activity of the tumor cells or fibroblasts in the inner or outer area of the tumor and LNM were examined by the t-test. The correlation between MVC in the inner or outer area of the tumor and LNM was also examined by the t-test. Then, the median values of proliferative activity of tumor cells or fibroblasts in the inner or outer area of the tumor with LNM were used as the cutoff value predicting LNM or DOM in this study. Similarly, the median cutoff values of MVC in the inner or outer area of the tumor with LNM were used for predicting LNM or DOM. MIB-1 LI or MVC of median value or lower was set up as low MIB-1 LI or low MVC, and MIB-1 LI or MVC higher than median value was high MIB-1 LI or high MVC.
The following variables were examined as potential predictive parameters for LNM or DOM: 1) proliferative activity of tumor cells in the inner or outer area; 2) proliferative activity of fibroblasts in the inner or outer area; 3) MVC in the inner or outer area; 4) invasive tumor size (≤20 mm versus >20 mm); 5) histological grade (1/2 versus 3); 6) tumor necrosis (absent versus present); 7) lymphatic invasion (absent versus present); 8) vascular invasion (absent versus present); 9) adipose tissue invasion (absent versus present); 10) nodal status (absent versus present); 11) c-erbB-2 expression (negative versus positive); and 12) ER expression (negative versus positive). Then the parameters that showed a significant correlation with LNM in the univariate analyses were entered into the logistic regression model 18 using the step-down method until all of the remaining factors were significant at a P value below 0.05. The Cox proportional hazard regression model was used to estimate the multivariate hazard rates (HRs) of DOM, with their 95% confidence interval (CI), 19 using the step-down method. In addition to the analysis of all cases (n = 204), the relative risks (RRs) for LNM or the HRs for DOM were computed for subgroups of cases in tumors ≤20 mm in size and in those 21 to 50 mm in size, respectively. Tumors >50 mm in size were not studied, because the number of such cases was small (n = 26). All analyses were conducted with Statistica/Windows software (StatSoft, Tulsa, OK).
Results
Correlation between Proliferative Activity of Tumor Cells and Fibroblasts
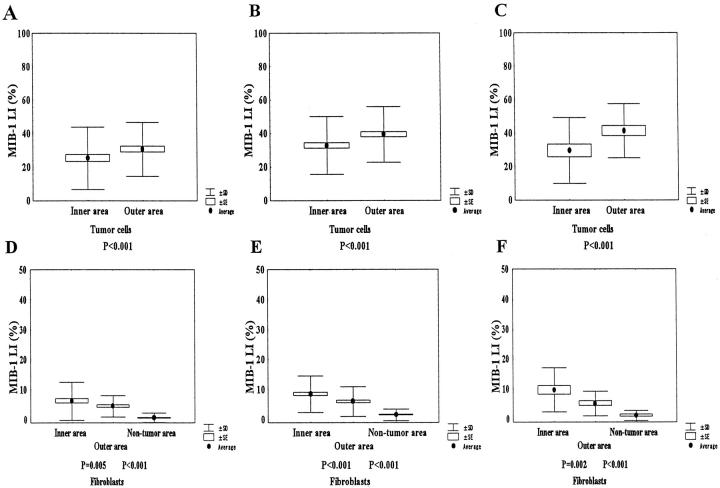
In all cases, the mean MIB-1 LI of tumor cells in the inner area was 29.8 ± 18.3% standard deviation (SD), and the mean MIB-1 LI of tumor cells in the outer area was 36.5 ± 16.8% SD. The latter was significantly higher than the former (P < 0.001). In contrast, the mean MIB-1 LI of fibroblasts in the inner area (7.9 ± 6.4% SD) was significantly higher than that in the outer area (5.4 ± 4.4% SD; P < 0.001). The mean MIB-1 LI (%) of fibroblasts in a nontumor area was 1.2 ± 1.8% SD, and was significantly lower than that of fibroblasts in the inner or outer areas (P < 0.001).
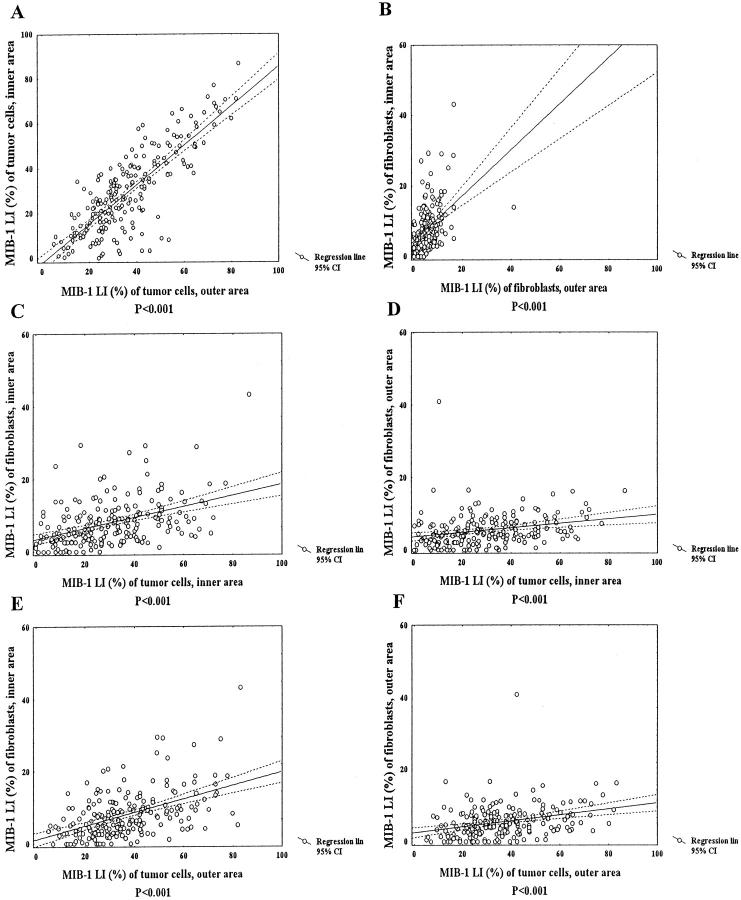
The MIB-1 LI of tumor cells in the inner area was significantly associated with the MIB-1 LI of tumor cells in the outer area (Figure 2A) ▶ . Similarly, there was a significant correlation between the MIB-1 LI of fibroblasts in the inner and outer areas (Figure 2B) ▶ . The MIB-1 LI of tumor cells in the inner area was significantly associated with that of fibroblasts in the inner or outer area (Figure 2 ▶ , C and D). There was also a significant association between the MIB-1 LI of tumor cells in the outer area and the MIB-1 LI of fibroblasts in the inner or outer area (Figure 2 ▶ , E and F). The MIB-1 LI of fibroblasts in the nontumor area was also significantly correlated with that of the MIB-1 LI of tumor cells in the inner (Pearson’s correlation coefficient: r = 0.36, P < 0.001) or outer area (Pearson’s correlation coefficient: r = 0.39, P < 0.001).
Figure 2.
Correlation between MIB-1 LI (%) of tumor cells and that of fibroblasts in the inner and outer areas. A: Significant correlation between MIB-1 LIs of tumor cells in the inner and outer areas is observed (Pearson’s correlation coefficient: r = 0.80, P < 0.001). B: There is a significant correlation between MIB-1 LIs of fibroblasts in the inner and outer areas (Pearson’s correlation coefficient: r = 0.45, P < 0.001). C and D: MIB-1 LI of tumor cells in the inner area is significantly associated with MIB-1 LI of fibroblasts in the inner or outer area (C, Pearson’s correlation coefficient: r = 0.43, P < 0.001; D, Pearson’s correlation coefficient: r = 0.25, P < 0.001). E and F: MIB-1 LI of tumor cells in the outer areas is significantly associated with MIB-1 LI of fibroblasts in the inner or outer area (E, Pearson’s correlation coefficient: r = 0.49, P < 0.001; F, Pearson’s correlation coefficient: r = 0.31, P < 0.001).
There were significant associations between the number of mitotic figures of tumor cells and the MIB-1 LIs of tumor cells in the inner and outer areas (Pearson’s correlation coefficient: r = 0.45, P < 0.001; and Pearson’s correlation coefficient: r = 0.54, P < 0.001, respectively). The MIB-1 LIs of fibroblasts in the inner and outer areas were also significantly associated with the number of mitotic figures in tumor cells (Pearson’s correlation coefficient: r = 0.23, P < 0.001; and Pearson’s correlation coefficient: r = 0.15, P = 0.036, respectively).
Correlation between Tumor Size and Proliferative Activity of Tumor Cells or Fibroblasts
When samples were separated according to tumor size, tumor cells in the outer area had significantly higher MIB-1 LIs than those in the inner area in tumors ≤20, 21 to 50, and >50 mm (Figure 3 ▶ , A–C).
Figure 3.
Proliferative activities of tumor cells or fibroblasts in the inner and outer areas in tumors of different sizes. A: Tumor cells in the outer area show significantly higher MIB-1 LI than those in the inner area in tumors ≤20 mm in size (P < 0.001). B and C: Tumor cells in the outer area still have significantly higher MIB-1 LI than those in the inner area in tumors 21 to 50, and >50 mm in size (P < 0.001). D–F: Fibroblasts in the inner area show significantly higher MIB-1 LIs than those in the outer area in tumors ≤20, 21 to 50, and >50 mm in size (P = 0.005, P < 0.001, and P = 0.002, respectively). Similarly, fibroblasts in the outer area show significantly higher MIB-1 LIs than those in nontumor area regardless of tumor size (P < 0.001).
The MIB-1 LIs of fibroblasts in the inner area were significantly higher than those of fibroblasts in the outer area regardless of tumor size (Figure 3 ▶ , D–F). Similarly, fibroblasts in the outer area had significantly higher MIB-1 LIs than those in the nontumor area regardless of tumor size (P < 0.001).
Correlations between Proliferative Activities of Tumor Cells or Fibroblasts and LNM
There was no significant correlation between mean MIB-1 LI of tumor cells in the inner (LNM-negative: 29.1 ± 19.7 SD, median = 24.4, versus LNM-positive: 31.2 ± 17.2 SD, median = 31.0; P = 0.424) or the outer area (LNM-negative: 34.5 ± 17.2 SD, median = 31.3, versus LNM-positive: 38.8 ± 16.7 SD, median = 35.6; P = 0.424), and LNM. Whereas, the mean MIB-1 LI of fibroblasts in the inner area of tumors with LNM was 9.8 ± 6.4 SD (median, 9.0), and that of tumors without LNM was 5.9 ± 6.0 SD (median, 4.6). The difference was significant (P < 0.001). In the outer area, the mean MIB-1 LI of fibroblasts with LNM was 6.2 ± 5.1 SD (median, 5.0), and that of fibroblasts without LNM was 4.6 ± 3.5 SD (median, 4.3). The former showed a significantly higher MIB-1 LI than the latter (P = 0.013). There was no significant correlation in mean MIB-1 LI of fibroblasts in nontumor area between tumors with (1.3 ± 1.9 SD) and without LNM (1.2 ± 1.8 SD) (P = 0.719).
The mean MVC of the inner area of tumors without LNM was 30.9 ± 17.1 SD (median, 26.5) and that of tumors with LNM was 35.2 ± 19.2 SD (median, 30.0). The difference was not significant (P = 0.106). In the outer area, the mean MVC of tumors without LNM was 36.3 ± 20.2 SD (median, 30.0) and that of tumors with LNM was 45.1 ± 31.6 SD (median, 41.0). The latter showed a significantly higher MVC than the former (P = 0.023).
The cutoff values of the MIB-1 LIs of tumor cells in the inner and outer areas were set at 30% and 35%, respectively, and those of fibroblasts in the inner and outer areas were set at 10% and 5%, respectively, based on the median MIB-1 LI of tumors with LNM. Tumors which had an MIB-1 LI of tumor cells or fibroblasts in the inner or outer area at or below the cutoff value were classified as the low MIB-1 LI group. All other tumors with MIB-1 LI values above the cutoff value formed the high MIB-1 LI group. The cutoff value of MVC in the inner and outer areas of the tumor was set at 30% and 40%, respectively, based on the median MVCs of tumors with LNM in both areas. Tumors with an inner or outer MVC at or below the cutoff value were classified as the low MVC group and those with a MVC more than the cutoff value formed the high MVC group.
Univariate and Multivariate Analyses for LNM
In all cases, the parameters which showed a significant correlation with LNM in univariate analyses were high MIB-1 LI of fibroblasts in the inner area, the presence of lymphatic or vascular invasion, high MVC of the outer areas, invasive tumor size >20 mm, positive c-erbB-2 expression, and the presence of adipose tissue invasion (Table 1 ▶ , Figure 4 ▶ ). Other parameters showed no significant correlation with LNM (data not shown). Multivariate analysis demonstrated that high MIB-1 LI of fibroblasts in the inner area, the presence of lymphatic invasion, and high MVC of the outer area still significantly increased the RRs of LNM (Table 1) ▶ . There was no significant increase in the RRs of LNM in invasive tumor size >20 mm, the presence of vascular invasion, positive c-erbB-2 expression, or the presence of adipose tissue invasion.
Table 1.
Univariate and Multivariate Analyses for LNM
| Parameters | Total | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| LNMR (%) | P | RR | 95% CI | P | ||
| All cases | 196 | |||||
| MIB-1 LI (%) of fibroblasts in inner area | ||||||
| Low | 143 | 59 (41) | Referent | |||
| High | 53 | 45 (85) | <0.001 | 9.2 | 3.8–22.2 | <0.001 |
| Lymphatic invasion | ||||||
| Absent | 106 | 41 (39) | Referent | |||
| Present | 90 | 63 (70) | <0.001 | 4.0 | 2.1–7.8 | <0.001 |
| MVC in outer area | ||||||
| Low | 110 | 51 (46) | Referent | |||
| High | 86 | 53 (61) | 0.034 | 2.4 | 1.2–4.0 | 0.011 |
| Invasive tumor size, mm | ||||||
| ≤20 | 72 | 26 (36) | Referent | |||
| >20 | 124 | 78 (63) | <0.001 | |||
| Vascular invasion | ||||||
| Absent | 149 | 72 (48) | Referent | |||
| Present | 47 | 32 (68) | 0.018 | |||
| c-erbB-2 expression | ||||||
| Negative | 115 | 30 (33) | Referent | |||
| Positive | 81 | 51 (63) | 0.020 | |||
| Adipose tissue invasion | ||||||
| Absent | 20 | 6 (30) | Referent | |||
| Present | 176 | 98 (56) | 0.029 | |||
| Invasive tumor size, mm | ||||||
| ≤20 cases | 70 | |||||
| MIB-1 LI (%) of fibroblasts in inner area | ||||||
| Low | 59 | 17 (29) | Referent | |||
| High | 11 | 8 (73) | 0.005 | 7.4 | 1.7–32.1 | 0.010 |
| Vascular invasion | ||||||
| Absent | 63 | 20 (32) | Referent | |||
| Present | 7 | 5 (71) | 0.038 | 6.5 | 1.7–32.1 | 0.044 |
| Tumor necrosis | ||||||
| Absent | 63 | 20 (32) | Referent | |||
| Present | 7 | 5 (71) | 0.038 | |||
| 21 to 50 cases | 103 | |||||
| MIB-1 LI (%) of fibroblasts in inner area | ||||||
| Low | 69 | 28 (41) | Referent | |||
| High | 34 | 29 (85) | <0.001 | 8.3 | 2.8–24.5 | <0.001 |
| Lymphatic invasion | ||||||
| Absent | 53 | 24 (45) | Referent | |||
| Present | 50 | 33 (66) | 0.036 |
LNMR, lymph node metastasis rate; RR, relative risk; CI, confidence interval; MVC, microvessel count; ER, estrogen receptor.
Univariate analyses for LNMR were performed by χ2 test. Multivariate analyses were performed by logistic regression model and adjusted with significantly correlated parameters with LNM listed on the table using the step-down method until all the remaining factors were significant at P < 0.05.
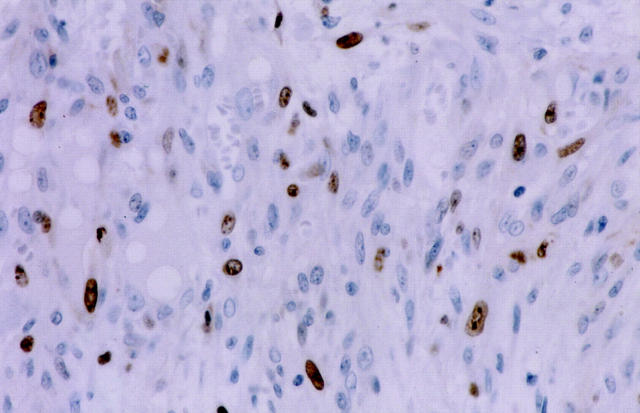
Figure 4.
Immunohistochemistry for MIB-1 of fibroblasts in the inner area within IDC. Many fibroblasts show positive nuclear staining for MIB-1.
In cases ≤20 mm in size, parameters which significantly increased the RRs of LNM in univariate analyses were high MIB-1 LI of fibroblasts in the inner area, the presence of vascular invasion, and the presence of tumor necrosis (Table 1) ▶ . Of these, high MIB-1 LI of fibroblasts in the inner area, and the presence of vascular invasion still significantly increased the RRs of LNM in multivariate analysis, but the presence of tumor necrosis failed to significantly increase the RRs of LNM. In cases with tumors 21 to 50 mm in size, univariate analyses showed that high MIB-1 LI of fibroblasts in the inner area, and the presence of lymphatic invasion were significantly correlated with LNM. In multivariate analysis, a significant increase of the RR of LNM was only observed in tumors with high MIB-1 LI of fibroblasts in the inner area (Table 1) ▶ . There was no significant correlation between other parameters and LNM in tumors ≤20 mm or those 21 to 50 mm in size (data not shown).
Univariate and Multivariate Analyses for DOM
In all cases, parameters which were significantly associated with DOM were high MIB-1 LI of fibroblasts in inner area, histological grade 3, the presence of LNM, high MIB-1 LI of tumor cells in the inner or outer area, negative ER expression, invasive tumor size ≤20 mm, and high MVC of the outer area (Table 2 ▶ , Figure 5A ▶ ). There was no significant correlation between DOM and c-erbB-2 expression, tumor necrosis, vascular invasion, MIB-1 LI of fibroblasts in the outer area, MVC in the inner area, lymphatic invasion, adipose tissue invasion, and type of operation (data not shown). Multivariate analysis showed that high MIB-1 LI of fibroblasts in the inner area, histological grade 3, and the presence of LNM significantly increased the HRs of DOM (Table 2) ▶ .
Table 2.
Univariate and Multivariate Analyses for DOM
| Parameters | Total | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| DOMR (%) | P | HR | 95% CI | P | ||
| All cases | 204 | |||||
| MIB-1 LI (%) of fibroblasts in inner area | ||||||
| Low | 150 | 13 (9) | Referent | |||
| High | 54 | 16 (30) | <0.001 | 2.9 | 1.4–6.5 | 0.007 |
| Histological grade | ||||||
| 1 and 2 | 142 | 10 (7) | Referent | |||
| 3 | 62 | 19 (31) | <0.001 | 4.9 | 2.2–10.6 | <0.001 |
| LNM | 196 | |||||
| Absent | 92 | 4 (4) | Referent | |||
| Present | 104 | 24 (23) | <0.001 | 4.0 | 1.3–12.2 | 0.013 |
| MIB-1 LI (%) of tumor cells in inner area | ||||||
| 204 | ||||||
| Low | 110 | 6 (5) | Referent | |||
| High | 94 | 23 (25) | <0.001 | |||
| MIB-1 LI (%) of tumor cells in outer area | ||||||
| Low | 111 | 6 (5) | Referent | |||
| High | 93 | 23 (25) | <0.001 | |||
| ER expression | ||||||
| Negative | 59 | 15 (25) | Referent | |||
| Positive | 145 | 14 (10) | 0.002 | |||
| Adjuvant therapy | ||||||
| No | 55 | 2 (3) | Referent | |||
| Yes | 149 | 27 (18) | 0.005 | |||
| Invasive tumor size, mm | ||||||
| ≤20 | 74 | 5 (7) | Referent | |||
| >20 | 130 | 24 (18) | 0.015 | |||
| MVC in outer area | ||||||
| Low | 112 | 11 (10) | Referent | |||
| High | 92 | 18 (20) | 0.046 | |||
| Invasive tumor size | ||||||
| 21 to 50 mm cases | ||||||
| 108 | ||||||
| MIB-1 LI (%) of fibroblasts in inner area | ||||||
| Low | 73 | 7 (10) | Referent | |||
| High | 35 | 12 (34) | <0.001 | 8.3 | 3.0–22.2 | <0.001 |
| MVC in outer area | ||||||
| Low | 55 | 4 (8) | Referent | |||
| High | 53 | 15 (28) | 0.003 | 11.0 | 3.4–35.7 | <0.001 |
| MIB-1 LI (%) of tumor cells in inner area | ||||||
| Low | 50 | 4 (8) | Referent | |||
| High | 58 | 15 (26) | 0.008 | 3.9 | 1.3–12.0 | 0.016 |
| Histological grade | ||||||
| 1 and 2 | 66 | 7 (11) | Referent | |||
| 3 | 42 | 12 (29) | 0.006 | |||
| MIB-1 LI (%) of tumor cells in outer area | ||||||
| Low | 50 | 4 (8) | Referent | |||
| High | 58 | 15 (26) | 0.008 | |||
| ER expression | ||||||
| Negative | 34 | 10 (29) | Referent | |||
| Positive | 74 | 9 (12) | 0.015 | |||
| LNM | ||||||
| Absent | 46 | 2 (4) | Referent | |||
| Present | 57 | 16 (23) | 0.016 | |||
| Adjuvant therapy | ||||||
| No | 25 | 1 (4) | Referent | |||
| Yes | 89 | 18 (22) | 0.022 |
DOMR, distant-organ metastasis rate; HR, hazard rate; CI, confidence interval; MVC, microvessel count; LNM, lymph node metastasis; ER, estrogen receptor.
Univariate analyses for DOMR were performed by log-rank test. Multivariate analyses were performed by Cox proportional hazard regression model and adjusted with parameters listed on the table using the step-down method until all the remaining factors were significant at P < 0.05.
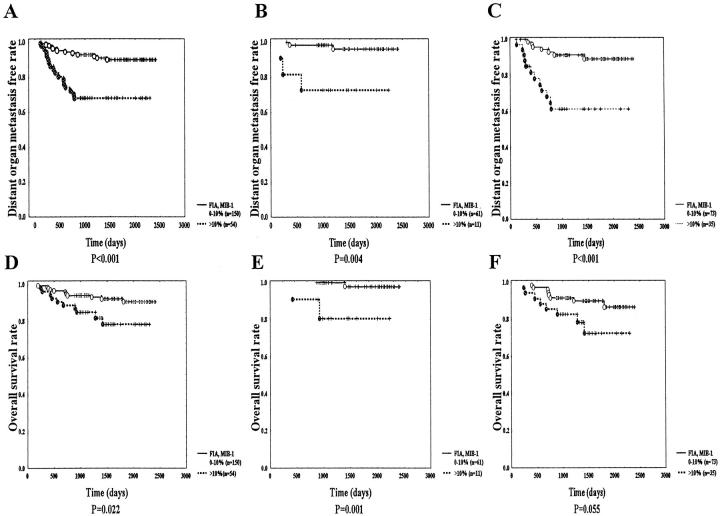
Figure 5.
DOM and OS curves by MIB-1 LI of fibroblasts in the inner area (FIA). A–C: IDCs with FIA MIB-1 LI > 10% show significantly shorter distant organ metastasis-free survivals in all cases, in tumors <20 mm, and those 21 to 50 mm in size than those with FIA MIB-1 LI ≤ 10% (P < 0.001, P = 0.004, and P < 0.001, respectively). D and E: In all cases and in cases ≤20 mm in size, tumors with FIA MIB-1 LI >10% show significantly shorter OS than those with FIA MIB-1 LI ≤ 10% (P = 0.022 and P = 0.001, respectively). F: In cases 21 to 50 mm in size, tumors with FIA MIB-1 LI > 10% show a marginally significantly shorter OS than those with FIA MIB-1 LI ≤ 10% (P = 0.055).
In cases ≤20 mm in size, high MIB-1 LI of fibroblast in the inner area, high MIB-1 LI of tumor cells in the inner or outer area, negative ER expression, the presence of tumor necrosis, and histological grade 3 were significantly associated with DOM (Figure 5B) ▶ . There was no significant correlation between other parameters and DOM (data not shown). Because there were only five cases with DOM, multivariate analysis could not be performed.
In cases 21 to 50 mm in size, there was a significant association of DOM with high MIB-1 LI of fibroblasts in the inner area, high MVC of the outer area, high MIB-1 LI of tumor cells in the inner or outer area, histological grade 3, negative ER expression and positive LNM (Table 2 ▶ , Figure 5C ▶ ). No other parameter showed any significant correlation with DOM (data not shown). In multivariate analysis, significant increases of the HRs of DOM were observed in tumors with high MIB-1 LI of fibroblasts in the inner area, those with high MVC of the outer area, and those with high MIB-1 LI of tumor cells in the inner area (Table 2) ▶ . Other parameters failed to significantly increase the HR of DOM.
As for OS, in all cases and in cases ≤20 mm, tumors with a high MIB-1 LI of fibroblasts in the inner area had a significantly shorter OS than those with a low MIB-1 LI of fibroblasts in the inner area (Figure 5 ▶ , D and E). In cases 21 to 50 mm in size, tumors with a high MIB-1 LI of fibroblasts in the inner area showed a marginally significant shorter OS than those with a low MIB-1 LI of fibroblasts in the inner area (Figure 5F) ▶ .
In all cases, other parameters significantly associated with OS were high MIB-1 LI of tumor cells in the inner or outer area, high MVC of the outer area, negative ER expression, invasive tumor size ≤20 mm, histological grade 3, positive LNM, and positive adjuvant therapy (P < 0.001, P < 0.001, P = 0.012, P < 0.001, P = 0.0016, P < 0.001, P < 0.001 and P = 0.013, respectively). No significant correlation with OS was observed in MIB-1 LI of fibroblasts in the outer area, MVC in the inner area, c-erbB-2 expression, tumor necrosis, lymphatic or vascular invasion, adipose tissue invasion, or type of operation (data not shown). Multivariate analysis showed significant associations of the HRs of OS with histological grade 3 (HR, 14.0; 95% CI, 4.1–47.2; P < 0.001) and positive LNM (HR, 4.6; 95% CI, 1.4–15.6; P = 0.014). High MIB-1 LI of fibroblasts in the inner area and other parameters failed to significantly increase the HRs of OS (data not shown). In tumors ≤20 mm in size, significant correlations with OS were observed in high MIB-1 LI of fibroblasts in the inner area, high MIB-1 LI of tumor cells in the inner or outer area, and histological grade 3 (P = 0.007, P = 0.016, and P < 0.001, respectively). Multivariate analysis could not be performed, because only three cases died of disease.
Discussion
Although many studies have evaluated the proliferative activity of tumor cells in breast cancer, 4-6,20 no report has shown a heterogeneity of proliferative activity of tumor cells within IDCs. In this study, it was clearly demonstrated that highly proliferative tumor cells are present in the outer area, and highly proliferative fibroblasts in the inner area of IDCs regardless of tumor size. Thus, this study clearly not only demonstrated that there is heterogeneity in proliferative activity of fibroblasts as well as that of tumor cells, but also that there is a discrepancy in the distribution of highly proliferative tumor cells and highly proliferative fibroblasts in IDCs. Therefore, proliferative activity of tumor cells must be assessed in tumor cells in the outer area, and that of fibroblasts must be assessed in fibroblasts in the inner area of IDCs.
In addition, this study also showed that proliferative activity of fibroblasts and that of tumor cells in the inner and outer areas were significantly associated with each other. This suggests that the tumor growth of IDCs does not depend only on proliferative activity of tumor cells, but also on that of fibroblasts.
This study clearly demonstrated that proliferative activity of fibroblasts in the inner area is the best parameter for prediction of LNM or DOM of IDC. The tumor-stromal interaction plays an important role in tumor invasion or metastasis. 7,8 Thus, this interaction may occur more actively in the inner area than the outer area within IDCs, and probably produces highly proliferative fibroblasts. This indicates that a paracrine mechanism of tumor progression in IDCs probably exists between tumor cells and fibroblasts in the inner area, and proliferative activity of fibroblasts may play a more important role than that of tumor cells in this interaction. However, some oncogenes can increase proliferation of fibroblasts, 21,22 and in fibroproliferative disease, such as scleroderma, proliferative fibroblasts express c-myb proto-oncogene. 23 Thus, there may be a different growth mechanism of fibroblasts in IDCs, and expression of some oncogene in fibroblasts may increase the proliferative activity of fibroblasts themselves. In addition, scleroderma or lung fibrosis is formed by proliferating fibroblasts escaping from apoptosis. 24,25 Therefore, it must be very important to clarify whether proliferation of intratumoral fibroblasts is dependent on the mechanism of apoptosis.
A significant enhancement of metastasis by highly proliferative fibroblasts in the inner area was clearly demonstrated in this study. It has been reported that fibroblasts within a tumor express proteinase, a very important determinant of tumor cell invasion or metastasis. 26,27 Therefore, it is suggested that highly proliferative fibroblasts, especially in the inner area of a tumor, secrete larger amounts of proteinases than lowly proliferative fibroblasts, which may increase the metastatic potential of IDCs.
Although the proliferative activity of fibroblasts in the inner area was the only parameter significantly associated with LNM or DOM, its effect on OS was inferior to that of histological grade three or that of positive LNM in multivariate analysis. Indeed, highly proliferative fibroblasts in the inner area are a component of IDC, but they are not neoplastic cells. Only tumor cells can proliferate destructively, leading to death of IDC patients. Therefore, highly proliferative fibroblasts in the inner area may provide a suitable environment for metastasis or aggressive growth of tumor cells, leading to mortality in IDC patients.
Breast cancer without nodal metastasis tends to involve marked reduction in the extent of tumor resection, 28,29 and it is very important to predict which IDCs have potential for LNM for reduction surgery. In this study, 80% or more of IDCs with highly proliferative fibroblasts in the inner area showed LNM, and in IDCs ≤20 mm in size, 73% of IDCs with highly proliferative fibroblasts in the inner area also showed LNM. Thus, proliferative activity of fibroblasts in the inner area may be a useful parameter regardless of tumor size at the time of decision of which IDC patients are to be treated with reduction surgery.
Acknowledgments
We thank Miss Megumi Horino for tissue preparation.
Footnotes
Address reprint requests to Dr. A. Ochiai, Pathology Division, National Cancer Center Research Institute East, Kashiwanoha 6–5-1, Kashiwa, Chiba 277-8577. E-mail: aochiai@east.ncc.go.jp.
Supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Health and Welfare and by a Grant-in-Aid for Second Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare in Japan.
A part of this work was presented at the 58th annual meeting of the Japanese Society of Cancer Research on September 29, 1999, in Hiroshima, Japan.
References
- 1.Fallenius AG, Auer GU, Carstensen JM: Prognostic significance of DNA measurements in 409 consecutive breast cancer patients. Cancer 1988, 62:331-341 [DOI] [PubMed] [Google Scholar]
- 2.MacGrogan G, Jollet I, Huet S, Sierankowski G, Picot V, Bonichon F, Coindre JM: Comparison of quantitative and semiquantitative methods of assessing MIB-1 with the S-phase fraction in breast carcinoma. Mod Pathol 1997, 10:769-776 [PubMed] [Google Scholar]
- 3.Thor AD, Liu S, Moore DH, II, Edgerton SM: Comparison of mitotic index, in vitro bromodeoxyuridine labeling, and MIB-1 assays to quantitate proliferation in breast cancer. J Clin Oncol 1999, 14:470-477 [DOI] [PubMed] [Google Scholar]
- 4.Cooper LS, Gillett CE, Fentiman IS, Barns DM: Cell proliferation measured by MIB1 and timing of surgery for breast cancer. Br J Cancer 1998, 77:1502-1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jansen RL, Hupperets PS, Arends JW, Joosten-Achjanie SR, Volovics A, Schouten HC, Hillen HF: MIB-1 labeling index is an independent prognostic marker in primary breast cancer. Br J Cancer 1998, 78:460-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biesterfeld S, Kluppel D, Koch R, Schneider S, Steinhagen G, Mihalcea AM, Schroder W: Rapid and prognostically valid quantification of immunohistochemical reactions by immunohistometry of the most positive tumour focus. A prospective follow-up study on breast cancer using antibodies against MIB-1, PCNA, ER and PR. J Pathol 1998, 185:25-31 [DOI] [PubMed] [Google Scholar]
- 7.Booth C, Harnden P, Trejdosiewicz LK, Scriven S, Selby PJ, Soughgate J: Stromal and vascular invasion in an human in vitro bladder cancer model. Lab Invest 1997, 76:843-857 [PubMed] [Google Scholar]
- 8.Uría JA, Stahle-Bäckdahl M, Seiki M, Fueyo A, Fueyo A, López-Otín C: Regulation of collagenase-3 expression in human breast carcinomas is mediated by stromal-epithelial cell interactions. Cancer Res 1997, 57:4882-4888 [PubMed] [Google Scholar]
- 9.Wright C, Angus B, Nicholson S, Sainsbury JR, Cairns J, Gullick WJ, Kelly P, Harris AL, Horne CH: Expression of c-erbB-2 oncoprotein: a prognostic indicator in human breast cancer. Cancer Res 1989, 49:2087-2090 [PubMed] [Google Scholar]
- 10.Weidner N, Semple JP, Welch WR, Folkman J: Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. New Engl J Med 1991, 324:1-8 [DOI] [PubMed] [Google Scholar]
- 11.Jitsuiki Y, Hasebe T, Tsuda H, Imoto S, Tsubono Y, Sasaki S, Mukai K: Optimizing microvessel counts according to tumor zone in invasive ductal carcinoma of the breast. Mod Pathol 1999, 12:492-498 [PubMed] [Google Scholar]
- 12.Sobin LH, Wittekind CH: TNM Classification of Malignant Tumors, 5th ed. 1997, :pp 93 Wiley-Liss, New York [Google Scholar]
- 13.: WHO: Histological typing of breast tumors. 2nd ed. International Histological Classification of Tumors. 1981, :pp 806 World Health Organization, Geneva, [Google Scholar]
- 14.Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410 [DOI] [PubMed] [Google Scholar]
- 15.Hsu SM, Raine L, Fanger H: The use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 16.Shi SR, Key ME, Kalza KL: Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991, 39:741-746 [DOI] [PubMed] [Google Scholar]
- 17.Hollingsworth CH, Kohn EC, Steinberg SM, Rothenberg ML, Merino MJ: Tumor angiogenesis in advanced stage ovarian carcinoma. Am J Pathol 1995, 147:33-41 [PMC free article] [PubMed] [Google Scholar]
- 18.Truett J, Cornfield J, Kannel W: A multivariate analysis of the risk of coronary heart disease in Framingham. J Chron Dis 1967, 20:511-524 [DOI] [PubMed] [Google Scholar]
- 19.Cox DR: Regression models and life-tables. J R Stat Soc 1972, 34:187-220 [Google Scholar]
- 20.Tynninen O, von Boguslawski K, Aronen HJ, Paavonen T: Prognostic value of vascular density and cell proliferation in breast cancer patients. Pathol Res Prac 1999, 195:31-37 [DOI] [PubMed] [Google Scholar]
- 21.Yan Y, Ouellette MM, Shay JW, Wright WE: Age-dependent alterations of c-fos and growth regulation in human fibroblasts expressing the HPV16 E6 protein. Mol Biol Cell 1996, 7:975-983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olrandini M, Semplici F, Erruzzi R, Meggio F, Pinna LA, Oliviero S: Protein kinase CK2alpha’ is induced by serum as a delayed early gene and cooperates with Ha-ras in fibroblast transformation. J Biol Chem 1998, 273:21291-21297 [DOI] [PubMed] [Google Scholar]
- 23.Piccinini G, Luchetti MM, Caniglia ML, Carossino AM, Montroni M, Introna M, Gabrielli A: c-myb proto-oncogene is expressed by quiescent scleroderma fibroblasts and, unlike B-myb gene, does not correlate with proliferation. J Invest Dermatol 1996, 106:1281-1286 [DOI] [PubMed] [Google Scholar]
- 24.Pablos JL, Carreira PE, Serrano L, Del Castillo P, Comez-Reino JJ: Apoptosis and proliferation of fibroblasts during postnatal skin development and scleroderma in the thight-skin mouse. J Histochem Cytochem 1997, 45:711-719 [DOI] [PubMed] [Google Scholar]
- 25.Guinee D, Jr, Brambilla E, Fleming M, Hayashi T, Rahn M, Koss M, Ferrans V, Travis W: The potential role of BAX and BCL-2 expression in diffuse alveolar damage. Am J Pathol 1997, 151:999-1007 [PMC free article] [PubMed] [Google Scholar]
- 26.Noel A, Hajitou A, L’Hoir C, Maquoi E, Baramova E, Lewalle JM, Remacle A, Kebers F, Brown P, Calberg-Bacq CM, Foidart JM: Inhibition of stromal matrix metalloproteases: effects on breast-tumor promotion by fibroblasts. Int J Cancer 1998, 76:267-273 [DOI] [PubMed] [Google Scholar]
- 27.Afzal S, Lalani EN, Poulsom R, Stubbs A, Rowlinson G, Sato H, Seiki M, Stamp GW: MT1-MMP and MMP-2 mRNA expression in human ovarian tumors: possible implications for the role of desmoplastic fibroblasts. Hum Pathol 1998, 29:155-165 [DOI] [PubMed] [Google Scholar]
- 28.Reynolds C, Mick R, Donohue JH, Grant CS, Farley DR, Callans LS, Orel SG, Keeney GL, Lawton TJ, Czerniecki BJ: Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol 1999, 17:1720-1726 [DOI] [PubMed] [Google Scholar]
- 29.Casey M, Rosenblatt R, Zimmerman J, Fineberg S: Mastectomy without malignancy after carcinoma diagnosed by large core stereotactic breast biopsy. Mod Pathol 1997, 10:1209-1213 [PubMed] [Google Scholar]