Figure 7.
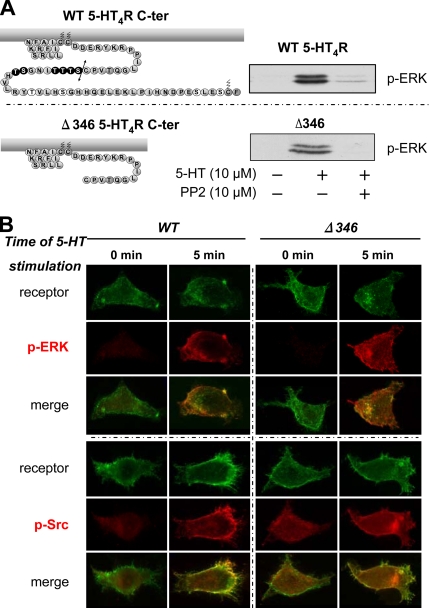
5-HT4R–mediated ERK phosphorylation through Src activation does not involve the C-terminal domain of 5-HT4R. (A) The C terminus is not implicated in Src-dependent 5-HT4R–mediated ERK activation. Topology of the C-terminal domain of 5-HT4R WT and Δ346 is represented on the left. Putative Ser/Thr phosphate acceptor sites are represented by black circles. HEK293 cells were transfected with 100 ng of Rho-tagged WT and Δ346 5-HT4R. Serum-starved HEK293 cells expressing the WT and the mutant were pretreated or not with the Src kinase inhibitor PP2 at 10 μM for 30 min before the stimulation with 10 μM 5-HT for 5 min. Whole cell lysates were prepared and analyzed by immunoblotting with antibody to p-ERK1/2. A representative blot of each experiment is shown. Both WT and truncated mutant Δ346 phosphorylate ERK1/2 in the same manner, dependent on Src kinase. (B) Colocalization of p-ERK1/2 and active p-Src after WT and Δ346 stimulation. HEK293 transfected with 500 ng of Rho-tagged 5-HT4R or Rho-tagged Δ346 were seeded onto coverslips. Twenty-four hours after transfection, cells were serum starved overnight. Cells were incubated 90 min with antibody against Rho-tagged 5-HT4R at 4°C before a 5- or 30-min stimulation period with 10 μM 5-HT. After fixation and permeabilization, the cells were sequentially incubated with primary antibody against p-ERK1/2 or p-Src (Tyr 416) and with fluorochrome-labeled secondary antibody. Fluorescence microscopy was then used to visualize distribution of antibody-labeled receptors (green channel) and appearance of the phosphorylated form of active ERK1/2 and active Src (red channel). Immunofluorescence microscopy was performed using a Zeiss Axiophot2 microscope with Zeiss 63× NA 1.4 oil immersion lenses. Representative images from several independent experiments are shown. Top, distribution of Rho-tagged 5-HT4R or Rho-tagged Δ346 before (basal) or after 5-min treatment with 10 μM 5-HT at 37°C. An increase in phosphorylation of p-ERK1/2 is visualized at the plasma membrane after 5-min stimulation with 5-HT of both WT and Δ346. Merged images were magnified to show colocalization of both receptors (WT and Δ346) with p-ERK1/2 at the plasma membrane after stimulation. Bottom, phosphorylation state of active Src before (basal) and after (5 min) 5-HT4R stimulation. Phosphorylation states were enhanced after stimulation with 5-HT and localization of p-ERK and p-Src do not differ between WT and Δ346 after 5-min stimulation.