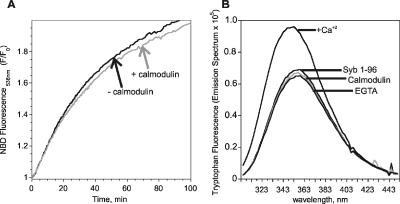
Figure 4.
Calmodulin binds to soluble synaptobrevin in a calcium-dependent manner but does not influence SNARE-mediated lipid mixing. Calmodulin binding to synaptobrevin was monitored by fluorescence emission of membrane-proximal tryptophan residues of synaptobrevin. (A) SNARE-mediated lipid mixing assay monitored by the lipid dequenching assay, as in Figure 2D. Ca2+/Calmodulin (1 μM) did not influence the rate of SNARE-mediated lipid mixing. Calcium (100 μM) alone had no effect on fusion (not shown). (B) Ca2+/Calmodulin binds synaptobrevin (Syb1–96). Tryptophan fluorescence emission derived from Syb 1–96 (1 μM) remained unchanged when calmodulin (1 μM) was added. Fluorescence emission increased and was slightly blue-shifted when calcium (100 μM) was added, which was reversed upon addition of EGTA (1 mM).