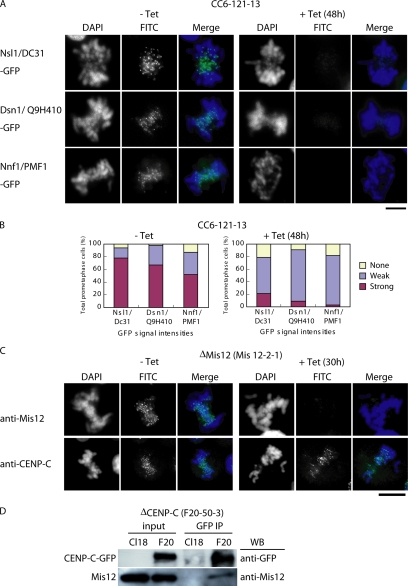
Figure 6.
Centromere localization of the Mis12 complex proteins is abolished in CENP-C–deficient cells. (A) To investigate localization of Mis12 complex proteins, plasmid constructs for Nsl1/DC31-GFP, Dsn1/Q9H410-GFP, and Nnf1/PMF1-GFP were transfected into CC6-121-13 cells. Localization of each GFP fusion protein (green) was observed in the absence (−tet) or presence (+tet) of tetracycline. DNA was counterstained with DAPI (blue). Bars, 10 μm. (B) Quantification of cells with various intensities of Nsl1-GFP, Dsn1-GFP, and Nnf1-GFP signals in CC6-121-13 cells at 0 h (−tet) or 48 h (+tet) after addition of tet. Average of intensity in the weak group is ∼20% of the level in the strong group. (C) Immunofluorescence analysis of Mis12-2-1 cells, which carry a conditional knockout of Mis12, with anti-Mis12 and anti-CENP-C antibodies. Antibody signals were detected with FITC-conjugated secondary antibodies (green). DNA was counterstained with DAPI (blue). Bars, 10 μm. (D) Coimmunoprecipitation of Mis12 protein with CENP-C-GFP. A cell line (F20-50-3) in which expression of CENP-C was replaced with that of CENP-C-GFP was created. The chromatin fractions of wild-type (Cl18) and F20-50-3 cells were immunoprecipitated with anti-GFP antibody. Immunoprecipitates were separated by SDS-PAGE and analyzed by Western blotting with anti-GFP and anti-Mis12 antibodies.