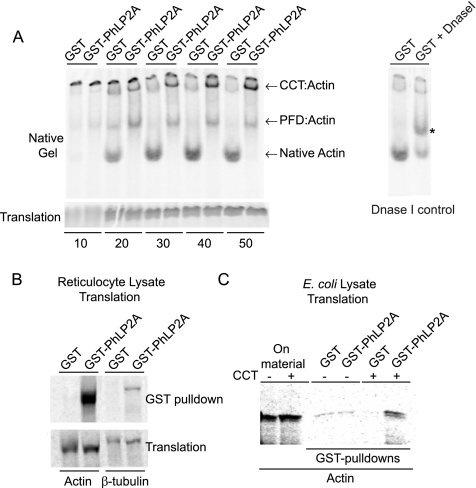
Figure 6.
Mammalian PhLP2A modulates CCT-mediated actin folding in vitro. (A) In vitro folding reactions of nascent 35S-labeled actin in the presence of GST or GST-PhLP2A and separated on a nondenaturing gel at the time points (minutes) indicated. CCT:actin and PFD:actin binary complexes, as well as native actin, are indicated. The identity of the fast-migrating band as native monomeric actin was verified by Dnase I-shifting on the native gel (right). Asterisk (*) indicates Dnase I bound native actin. Note the reduction in the intensity of the bottom band. Note the decrease in native actin production over time in the presence of GST-PhLP2A but not GST alone (see Supplemental Figure S3 for quantitation of band intensities). An SDS-gel illustrating the relative amounts of translation products is shown (bottom). (B) Coprecipitation of 35S-labeled actin or β-tubulin with GST-PhLP2A in a reticulocyte translation reaction. The lower panel shows that the levels of translation were comparable. (C) Coprecipitation of 35S-labeled actin produced in E. coli lysate takes place only in the presence of GST-PhLP2A and exogenously added CCT, indicating the formation of a ternary complex. Actin cDNA was translated in E. coli lysate with or without the addition of purified rabbit CCT, and the relative levels of the translation products are shown on the left. GST-PhLP2A coprecipitated the newly-made actin only in reactions to which CCT had been added, whereas GST alone had no such affect as shown on the right.