Abstract
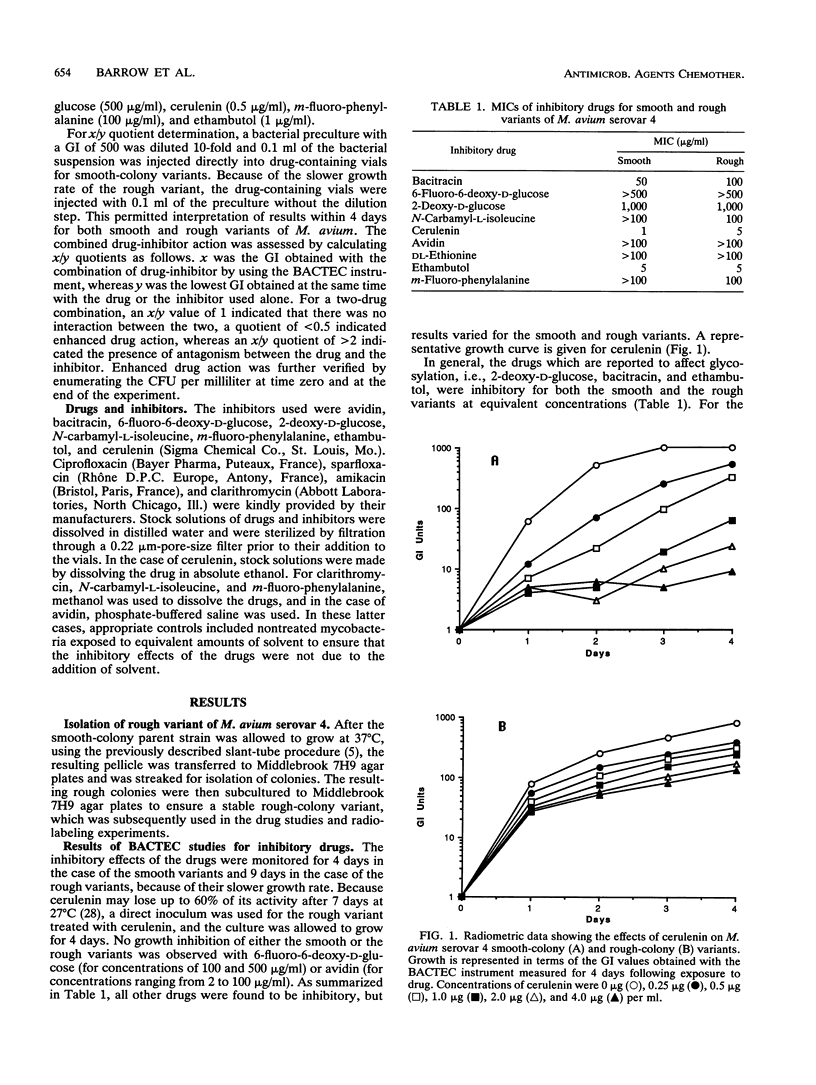
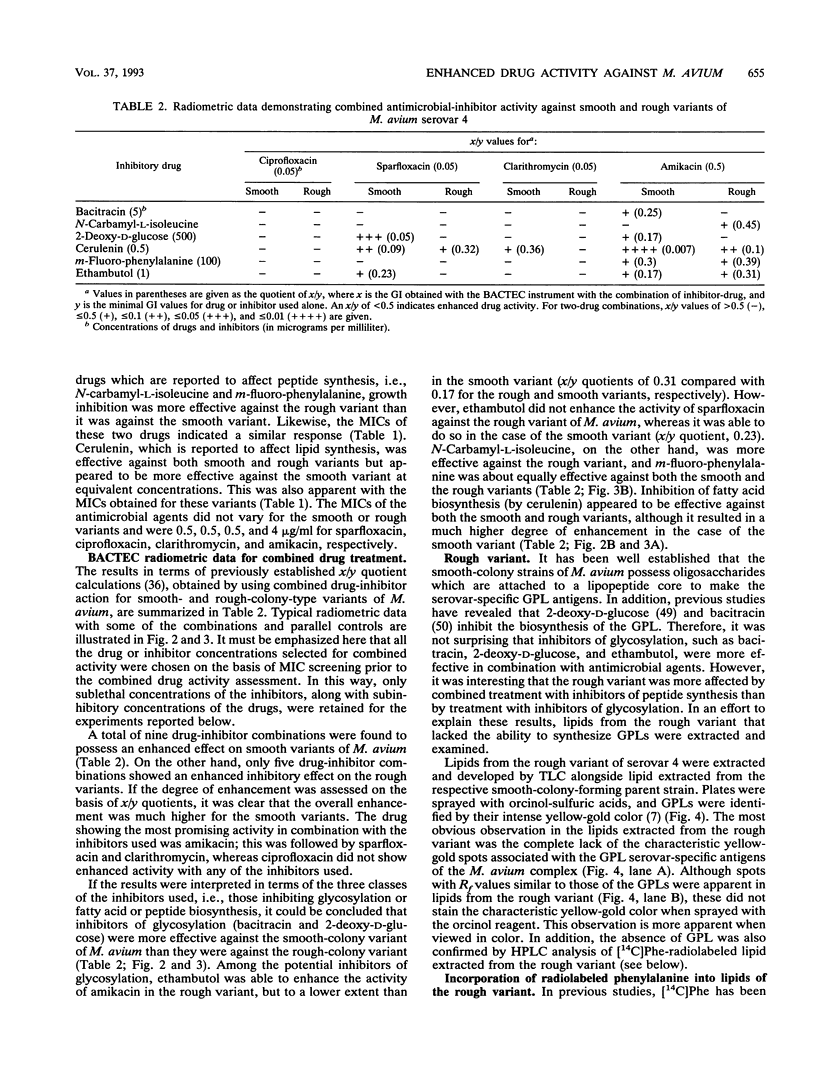
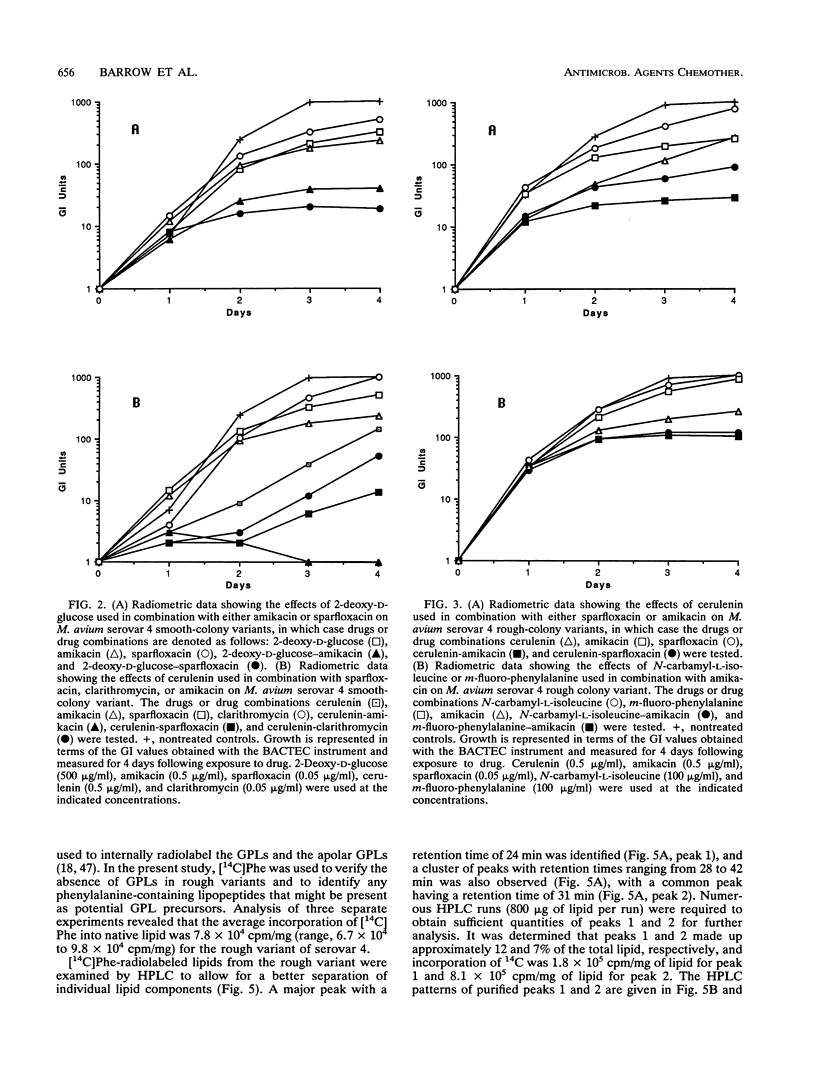
Smooth- and rough-colony variants of Mycobacterium avium serovar 4 were treated with three classes of drugs. The drugs were chosen for their potential inhibitory effects on the biosynthesis of the cell envelope-associated serovar-specific glycopeptidolipid antigens. Growth was monitored radiometrically with a BACTEC 460-TB instrument, and MICs were determined for each drug. Both variants were then treated with inhibitory drugs in combination with antimicrobial agents that have demonstrated effectiveness against M. avium. No growth inhibition was observed with 6-fluoro-6-deoxy-D-glucose or avidin. Inhibitors of glycosylation, i.e., 2-deoxy-D-glucose, bacitracin, and ethambutol, were inhibitory to smooth- and rough-colony variants, whereas drugs that inhibit peptide synthesis, i.e., N-carbamyl-L-isoleucine and m-fluoro-phenylalanine, were more inhibitory for the rough-colony variant. Cerulenin, which affects fatty acid synthesis, was inhibitory for both variants, but it appeared to be more effective at inhibiting the growth of the smooth-colony variant at equivalent concentrations. Generally, when inhibitors of glycosylation were used with sparfloxacin and amikacin, a synergistic effect was observed for only the smooth variant. When drugs that affect peptide synthesis were used in combination with amikacin, a synergistic effect was observed for the rough variant, and when cerulenin was used in combination with sparfloxacin or amikacin, a synergistic effect was observed for both variants. Lipid analysis revealed that although the rough variant lacks the serovar-specific glycopeptidolipid antigens, it does possess a group of phenylalanine-isoleucine-containing lipopeptides that may explain its different susceptibility patterns to m-fluoro-phenylalanine and N-carbamyl-L-isoleucine. The significance of these results is discussed with reference to various components in the cell envelope and their importance in cell wall permeability.
Full text
PDF

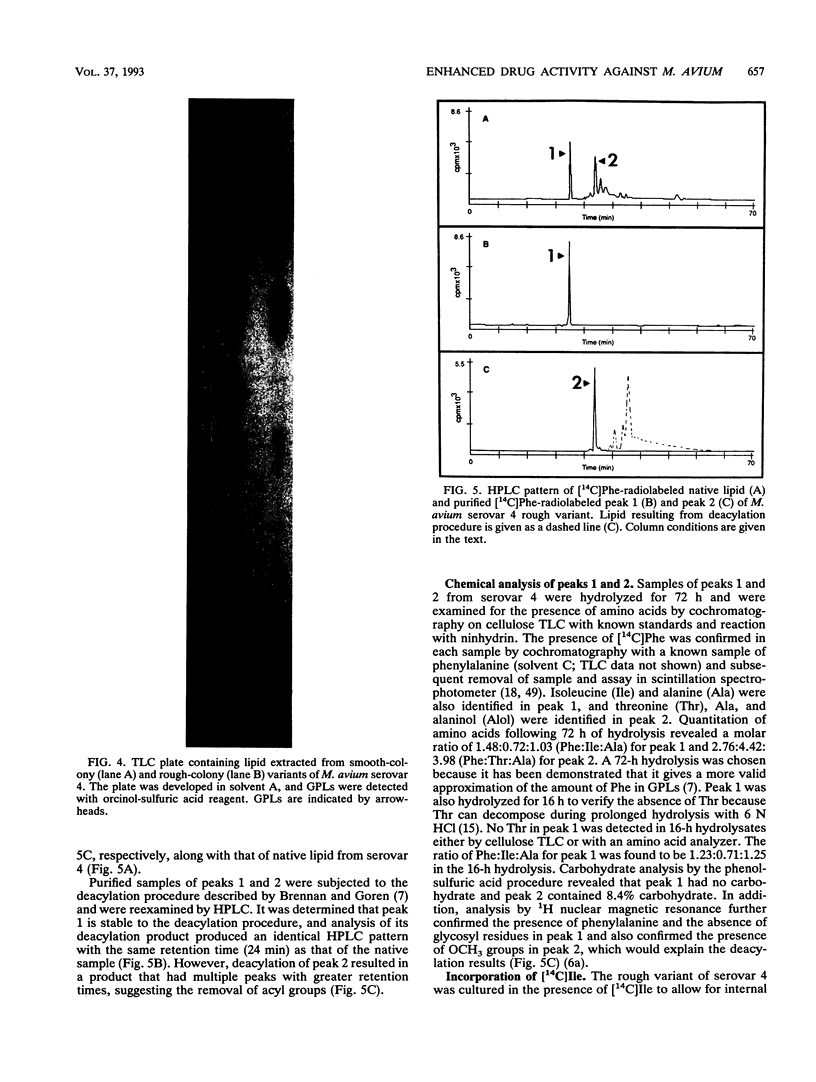
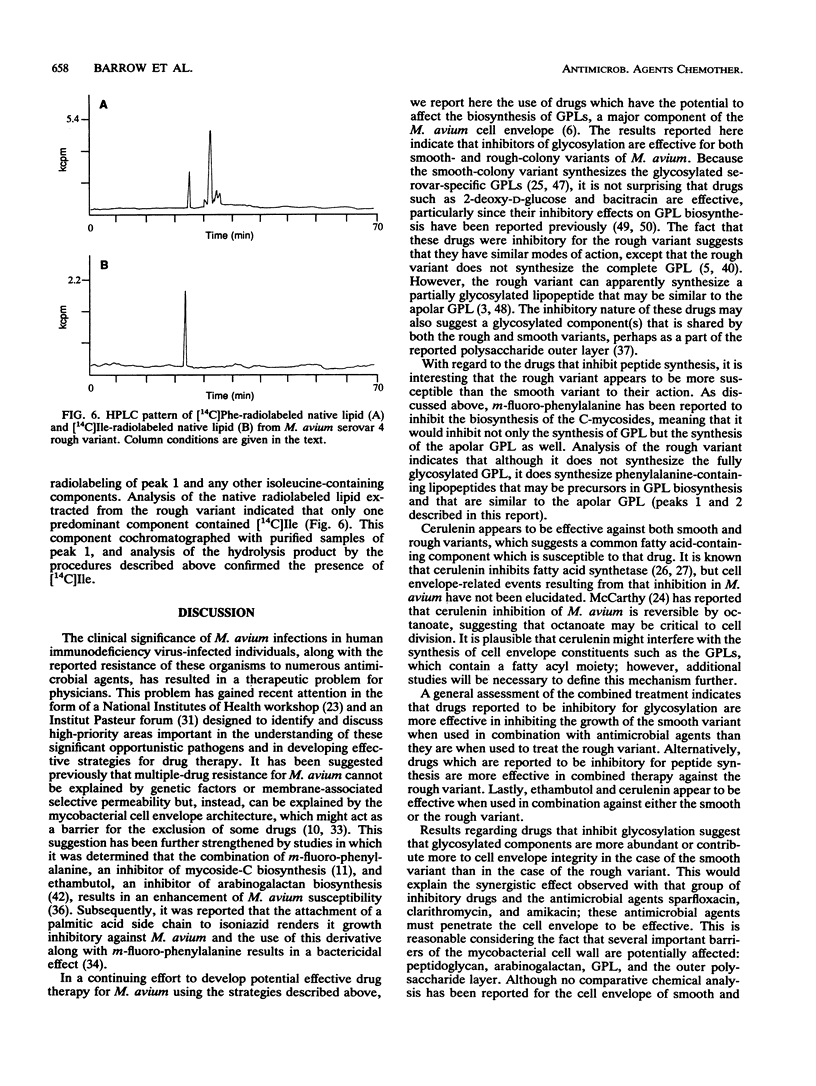



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agins B. D., Berman D. S., Spicehandler D., el-Sadr W., Simberkoff M. S., Rahal J. J. Effect of combined therapy with ansamycin, clofazimine, ethambutol, and isoniazid for Mycobacterium avium infection in patients with AIDS. J Infect Dis. 1989 Apr;159(4):784–787. doi: 10.1093/infdis/159.4.784. [DOI] [PubMed] [Google Scholar]
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Brennan P. J. Immunogenicity of type-specific C-mycoside glycopeptidolipids of mycobacteria. Infect Immun. 1982 May;36(2):678–684. doi: 10.1128/iai.36.2.678-684.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W., Brennan P. J. Isolation in high frequency of rough variants of Mycobacterium intracellulare lacking C-mycoside glycopeptidolipid antigens. J Bacteriol. 1982 Apr;150(1):381–384. doi: 10.1128/jb.150.1.381-384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow W. W. Contributing factors of pathogenesis in the Mycobacterium avium complex. Res Microbiol. 1991 May;142(4):427–433. doi: 10.1016/0923-2508(91)90115-q. [DOI] [PubMed] [Google Scholar]
- Barrow W. W., Ullom B. P., Brennan P. J. Peptidoglycolipid nature of the superficial cell wall sheath of smooth-colony-forming mycobacteria. J Bacteriol. 1980 Nov;144(2):814–822. doi: 10.1128/jb.144.2.814-822.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Chiu J., Nussbaum J., Bozzette S., Tilles J. G., Young L. S., Leedom J., Heseltine P. N., McCutchan J. A. Treatment of disseminated Mycobacterium avium complex infection in AIDS with amikacin, ethambutol, rifampin, and ciprofloxacin. California Collaborative Treatment Group. Ann Intern Med. 1990 Sep 1;113(5):358–361. doi: 10.7326/0003-4819-113-5-358. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Elkins N., May M. H. Effectiveness of ofloxacin against Mycobacterium tuberculosis and Mycobacterium avium, and rifampin against M. tuberculosis in cultured human macrophages. Am Rev Respir Dis. 1988 May;137(5):1141–1146. doi: 10.1164/ajrccm/137.5.1141. [DOI] [PubMed] [Google Scholar]
- David H. L. Basis for lack of drug susceptibility of atypical mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):878–884. doi: 10.1093/clinids/3.5.878. [DOI] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Draper P. The mycoside capsule of Mycobacterium Avium 357. J Gen Microbiol. 1974 Aug;83(2):431–433. doi: 10.1099/00221287-83-2-431. [DOI] [PubMed] [Google Scholar]
- Etzkorn E. T., Aldarondo S., McAllister C. K., Matthews J., Ognibene A. J. Medical therapy of Mycobacterium avium-intracellulare pulmonary disease. Am Rev Respir Dis. 1986 Sep;134(3):442–445. doi: 10.1164/arrd.1986.134.3.442. [DOI] [PubMed] [Google Scholar]
- Fukunishi Y., Okada S., Nishiura M., Kohsaka K. Ultrastructural features of the multiplication of human and murine leprosy bacilli in macrophages of nude mice. Int J Lepr Other Mycobact Dis. 1982 Mar;50(1):68–75. [PubMed] [Google Scholar]
- Hooper L. C., Johnson M. M., Khera V. R., Barrow W. W. Macrophage uptake and retention of radiolabeled glycopeptidolipid antigens associated with the superficial L1 layer of Mycobacterium intracellulare serovar 20. Infect Immun. 1986 Oct;54(1):133–141. doi: 10.1128/iai.54.1.133-141.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Hoy J., Mijch A., Sandland M., Grayson L., Lucas R., Dwyer B. Quadruple-drug therapy for Mycobacterium avium-intracellulare bacteremia in AIDS patients. J Infect Dis. 1990 Apr;161(4):801–805. doi: 10.1093/infdis/161.4.801. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Salton M. R., Barksdale L. Ultrastructure of superficial mycosidic integuments of Mycobacterium sp. J Bacteriol. 1976 Feb;125(2):739–743. doi: 10.1128/jb.125.2.739-743.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughon B. E., Allaudeen H. S., Becker J. M., Current W. L., Feinberg J., Frenkel J. K., Hafner R., Hughes W. T., Laughlin C. A., Meyers J. D. From the National Institutes of Health. Summary of the workshop on future directions in discovery and development of therapeutic agents for opportunistic infections associated with AIDS. J Infect Dis. 1991 Aug;164(2):244–251. doi: 10.1093/infdis/164.2.244. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Nomura S., Horiuchi T., Omura S., Hata T. The action mechanism of cerulenin. I. Effect of cerulenin on sterol and fatty acid biosynthesis in yeast. J Biochem. 1972 May;71(5):783–796. doi: 10.1093/oxfordjournals.jbchem.a129827. [DOI] [PubMed] [Google Scholar]
- Omura S. Cerulenin. Methods Enzymol. 1981;72:520–532. [PubMed] [Google Scholar]
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976 Sep;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasesh N., Wright E. L., Barrow W. W. Cell-free system responsible for internal radiolabeling of glycopeptidolipids of the Mycobacterium avium complex. Infect Immun. 1992 Jan;60(1):308–311. doi: 10.1128/iai.60.1.308-311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S. Action of 1-isonicotinyl-2-palmitoyl hydrazine against the Mycobacterium avium complex and enhancement of its activity by m-fluorophenylalanine. Antimicrob Agents Chemother. 1990 Nov;34(11):2061–2064. doi: 10.1128/aac.34.11.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S., David H. L. Drug susceptibility testing in tuberculosis: a comparison of the proportion methods using Lowenstein-Jensen, Middlebrook 7H10 and 7H11 agar media and a radiometric method. Res Microbiol. 1989 Jul-Aug;140(6):405–417. doi: 10.1016/0923-2508(89)90016-8. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S., David H. L. Enhancement of drug susceptibility of Mycobacterium avium by inhibitors of cell envelope synthesis. Antimicrob Agents Chemother. 1990 May;34(5):759–764. doi: 10.1128/aac.34.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Hellio R. Evidence that the capsule around mycobacteria grown in axenic media contains mycobacterial antigens: implications at the level of cell envelope architecture. FEMS Microbiol Lett. 1990 Jul;58(2):161–166. doi: 10.1111/j.1574-6968.1990.tb13971.x. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V. Extracellular and intracellular activities of clarithromycin used alone and in association with ethambutol and rifampin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991 Mar;35(3):462–470. doi: 10.1128/aac.35.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V., Goh K. S., De Sousa J. P. Antimycobacterial spectrum of sparfloxacin and its activities alone and in association with other drugs against Mycobacterium avium complex growing extracellularly and intracellularly in murine and human macrophages. Antimicrob Agents Chemother. 1991 Dec;35(12):2473–2480. doi: 10.1128/aac.35.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Levy-Frebault V., Blom-Potar M. C., David H. L. Ability of smooth and rough variants of Mycobacterium avium and M. intracellulare to multiply and survive intracellularly: role of C-mycosides. Zentralbl Bakteriol Mikrobiol Hyg A. 1989 Jan;270(3):345–360. doi: 10.1016/s0176-6724(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Rastogi N. Recent observations concerning structure and function relationships in the mycobacterial cell envelope: elaboration of a model in terms of mycobacterial pathogenicity, virulence and drug-resistance. Res Microbiol. 1991 May;142(4):464–476. doi: 10.1016/0923-2508(91)90121-p. [DOI] [PubMed] [Google Scholar]
- Rulong S., Aguas A. P., da Silva P. P., Silva M. T. Intramacrophagic Mycobacterium avium bacilli are coated by a multiple lamellar structure: freeze fracture analysis of infected mouse liver. Infect Immun. 1991 Nov;59(11):3895–3902. doi: 10.1128/iai.59.11.3895-3902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Kilburn J. O. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1989 Sep;33(9):1493–1499. doi: 10.1128/aac.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassell S. K., Pourshafie M., Wright E. L., Richmond M. G., Barrow W. W. Modified lymphocyte response to mitogens induced by the lipopeptide fragment derived from Mycobacterium avium serovar-specific glycopeptidolipids. Infect Immun. 1992 Feb;60(2):706–711. doi: 10.1128/iai.60.2.706-711.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- Woodbury J. L., Barrow W. W. Radiolabelling of Mycobacterium avium oligosaccharide determinant and use in macrophage studies. J Gen Microbiol. 1989 Jul;135(7):1875–1884. doi: 10.1099/00221287-135-7-1875. [DOI] [PubMed] [Google Scholar]
- Wright E. L., Barrow W. W. Inhibition of glycopeptidolipid synthesis resulting from treatment of Mycobacterium avium with 2-deoxy-D-glucose. Res Microbiol. 1991 Jun;142(5):597–608. doi: 10.1016/0923-2508(91)90193-e. [DOI] [PubMed] [Google Scholar]
- Yeager H., Jr, Raleigh J. W. Pulmonary disease due to Mycobacterium intracellulare. Am Rev Respir Dis. 1973 Sep;108(3):547–552. doi: 10.1164/arrd.1973.108.3.547. [DOI] [PubMed] [Google Scholar]