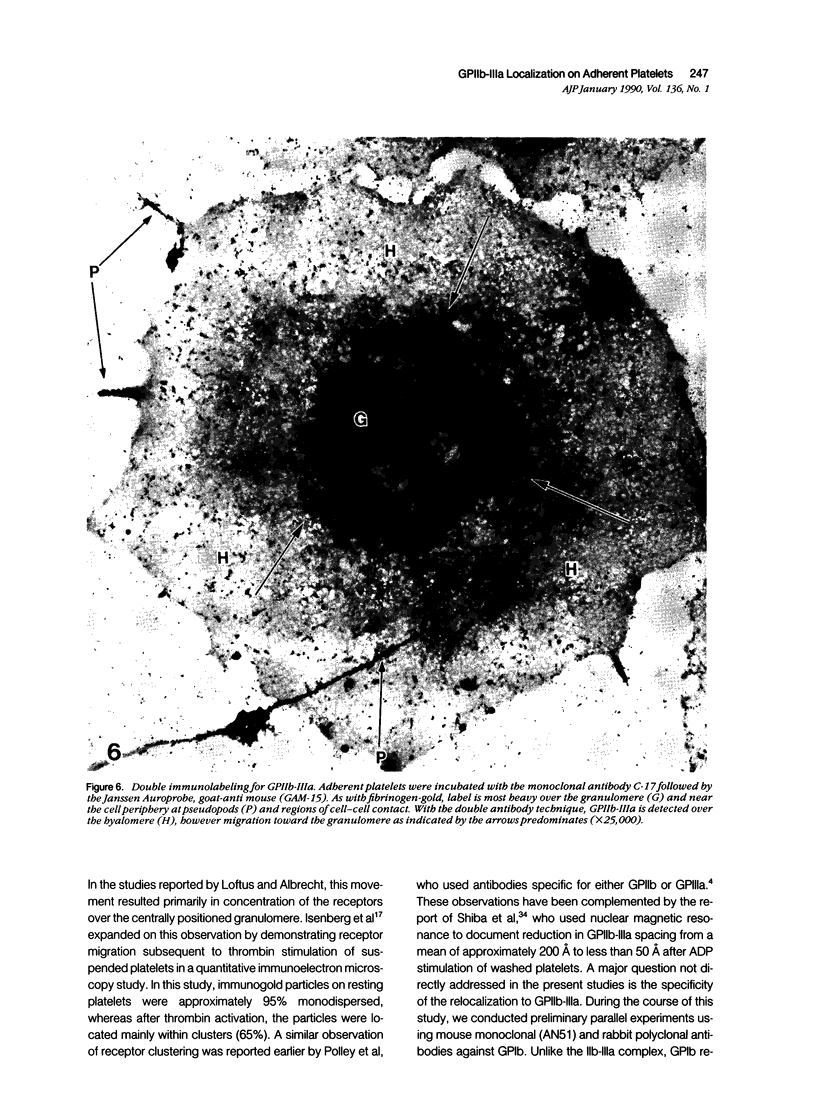
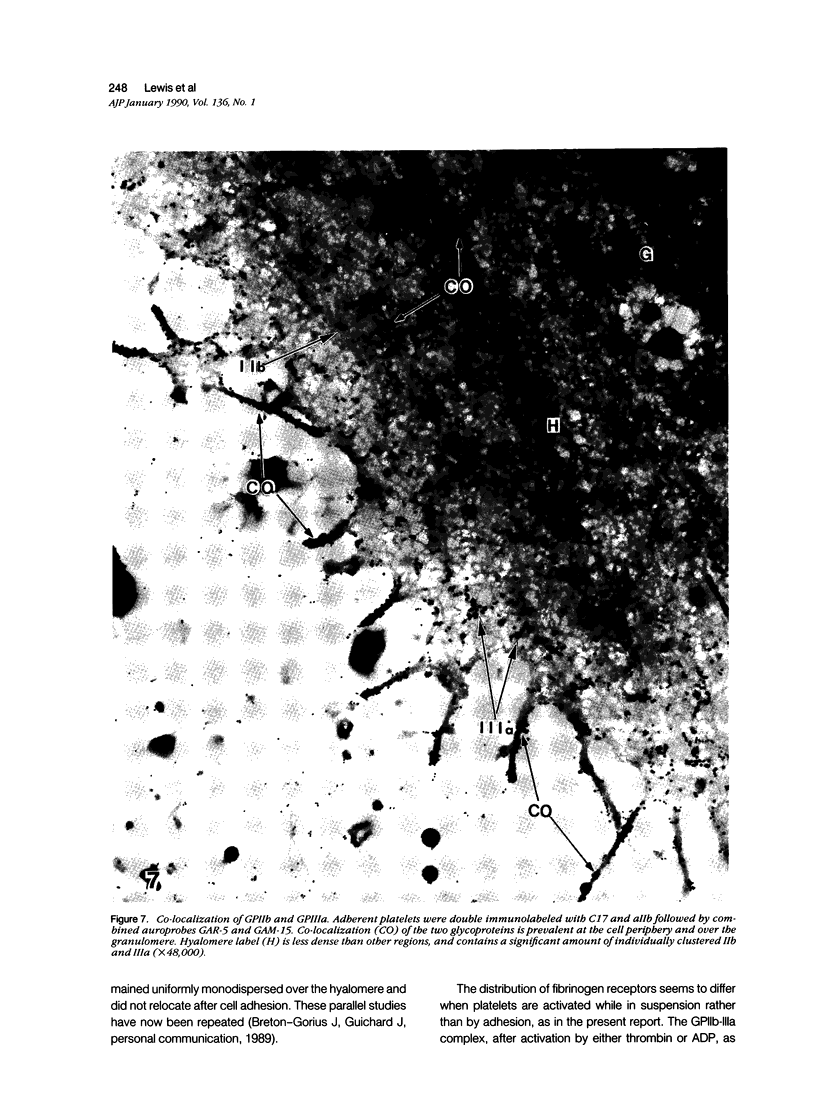
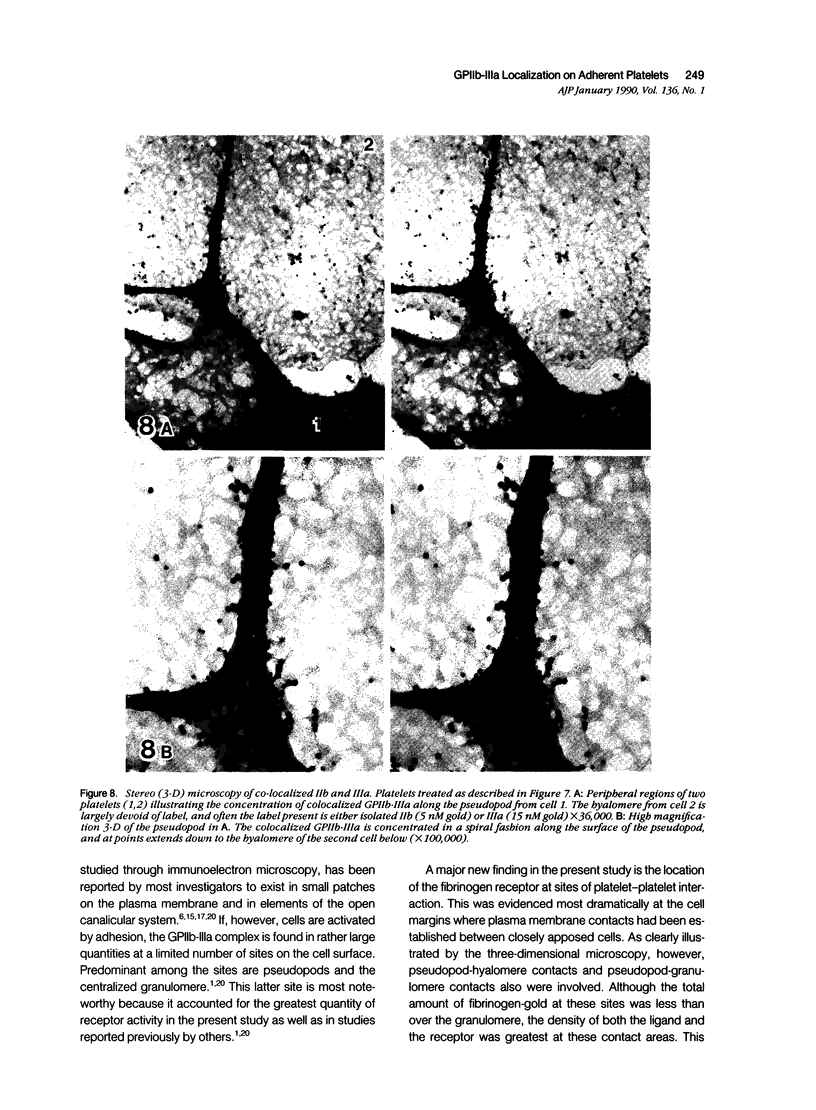
Abstract
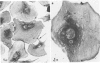
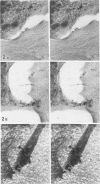
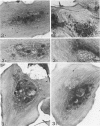
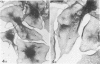
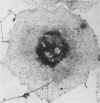
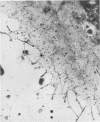
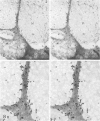
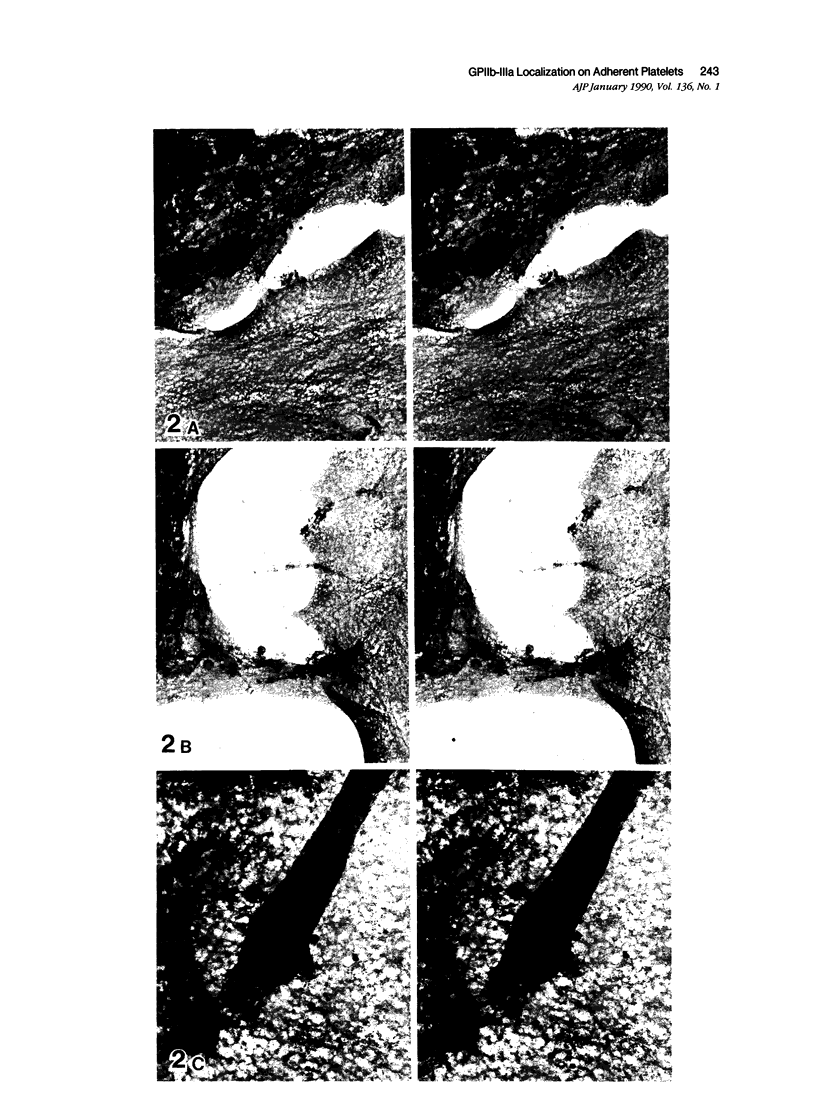
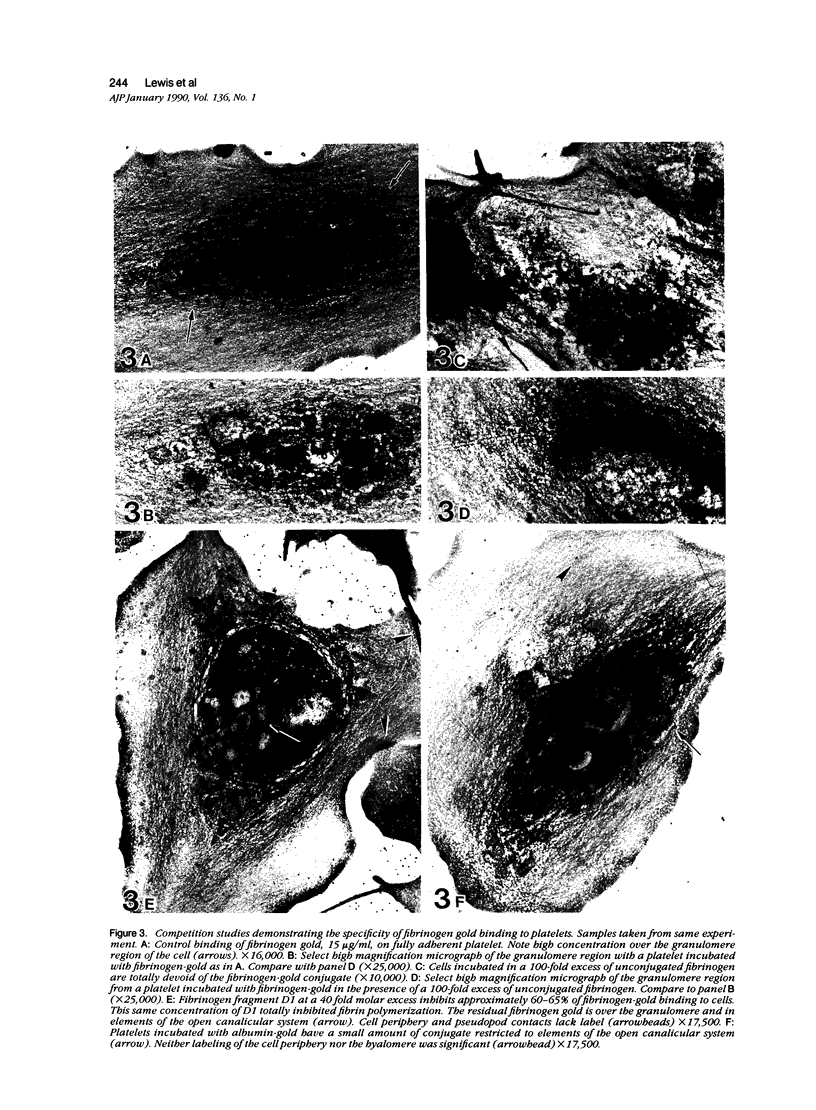
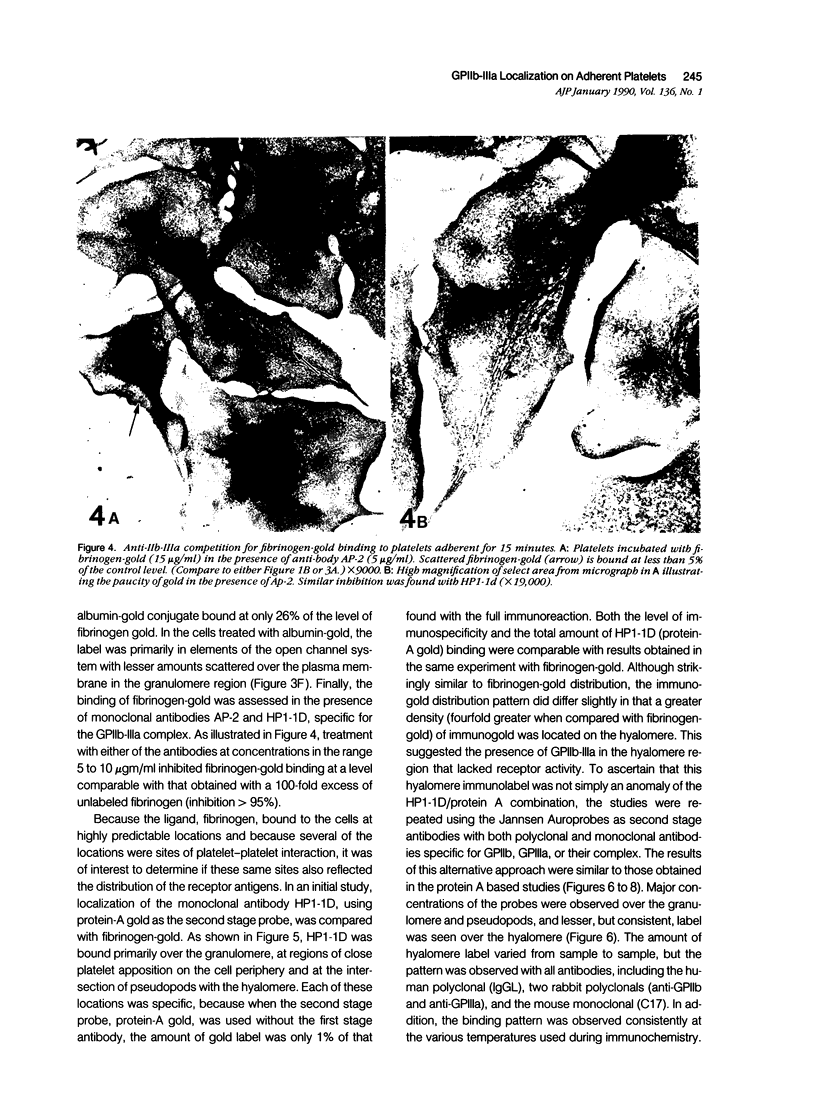
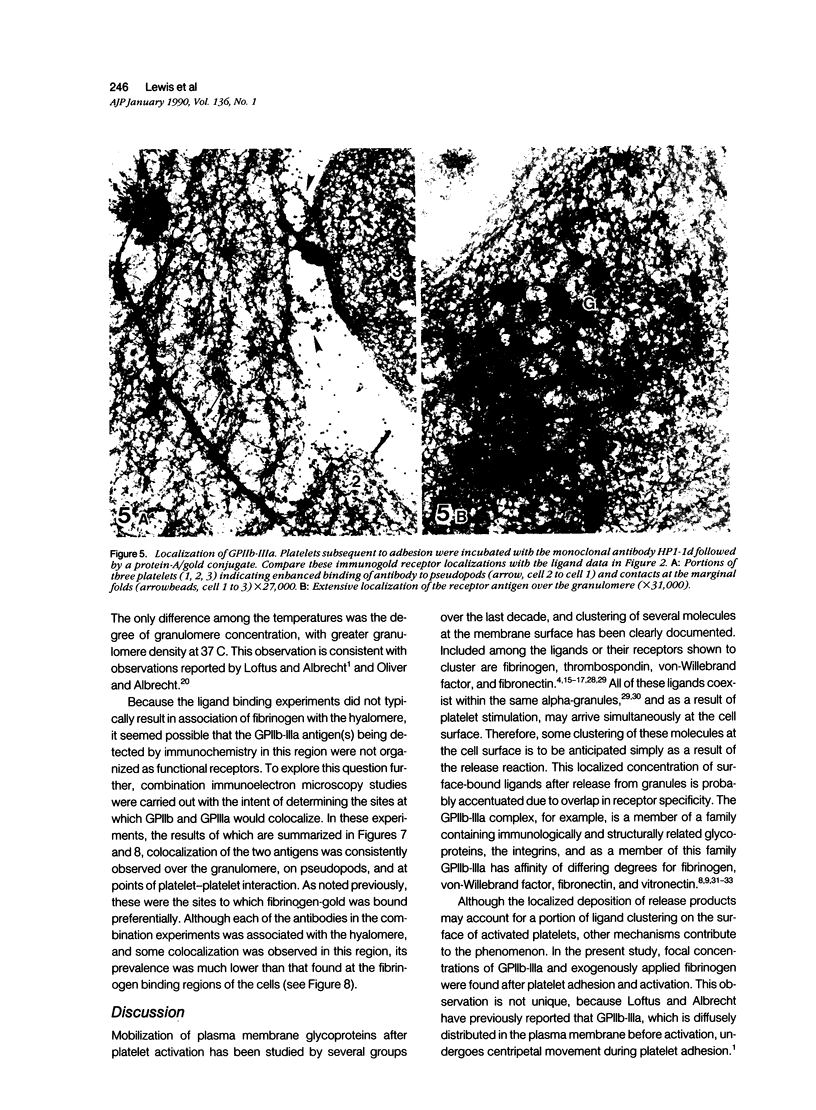
Platelet membrane glycoprotein IIb-IIIa plays a focal role in primary hemostasis by serving as the cell surface receptor for fibrinogen. Recent studies by several groups have suggested that GPIIb-IIIa, which is dispersed randomly in the resting cell, undergoes migration leading to receptor clustering after platelet activation. The authors have investigated this activation-dependent relocation of fibrinogen receptors on platelets adherent to a standardized artificial surface. The correlative use of immunogold electron microscopy, ligand-gold binding, and stereo (three-dimensional) electron microscopy (EM) revealed specific localization of fibrinogen and its receptor at points of platelet to platelet interaction. Fibrinogen distribution on the plasma membrane, studied through the use of fibrinogen-gold conjugates with whole-mount adherent platelets, was primarily over the granulomere and at the cell periphery corresponding to sites of platelet-platelet interaction. Compared with the general hyalomere, fibrinogen density over the granulomere and at contact regions was increased 12-fold and 22-fold, respectively, and the specificity of binding at these sites was verified by positive competition with native fibrinogen, one of its degradation products (Fragment D1), and by monoclonal antibodies (HP1-1d and AP-2) specific for GPIIb-IIIa. The distribution of receptor antigens, localized by immunogold EM using antibodies against GPIIb-IIIa, also was localized over the hyalomere, where fibrinogen did not bind. To understand this apparently nonfunctional hyalomere GPIIb-IIIa further, correlative immunocytochemistry was performed using polyclonal and monoclonal antibodies for GPIIb and GPIIIa simultaneously. Colocalization of the antigens was observed consistently over the granulomere and at regions of cell contact, whereas the hyalomere antigens tended to be nonassociated. The studies document GPIIb-IIIa as a function complex at sites of cell interaction where fibrinogen binds.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Leung L. L., Polley M. J., Nachman R. L. Platelet membrane topography: colocalization of thrombospondin and fibrinogen with the glycoprotein IIb-IIIa complex. Blood. 1985 Oct;66(4):926–934. [PubMed] [Google Scholar]
- Bray P. F., Rosa J. P., Lingappa V. R., Kan Y. W., McEver R. P., Shuman M. A. Biogenesis of the platelet receptor for fibrinogen: evidence for separate precursors for glycoproteins IIb and IIIa. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1480–1484. doi: 10.1073/pnas.83.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Gorius J., Lewis J. C., Guichard J., Kieffer N., Vainchenker W. Monoclonal antibodies specific for human platelet membrane glycoproteins bind to monocytes by focal absorption of platelet membrane fragments: an ultrastructural immunogold study. Leukemia. 1987 Feb;1(2):131–141. [PubMed] [Google Scholar]
- CROSS M. J. EFFECT OF FIBRINOGEN ON THE AGGREGATION OF PLATELETS BY ADENOSINE DIPHOSPHATE. Thromb Diath Haemorrh. 1964 Dec 31;12:524–527. [PubMed] [Google Scholar]
- Charo I. F., Fitzgerald L. A., Steiner B., Rall S. C., Jr, Bekeart L. S., Phillips D. R. Platelet glycoproteins IIb and IIIa: evidence for a family of immunologically and structurally related glycoproteins in mammalian cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer E. M., Caen J. P., Drouet L., Breton-Gorius J. Absence of tubular structures and immunolabeling for von Willebrand factor in the platelet alpha-granules from porcine von Willebrand disease. Blood. 1986 Sep;68(3):774–778. [PubMed] [Google Scholar]
- Duperray A., Berthier R., Chagnon E., Ryckewaert J. J., Ginsberg M., Plow E., Marguerie G. Biosynthesis and processing of platelet GPIIb-IIIa in human megakaryocytes. J Cell Biol. 1987 Jun;104(6):1665–1673. doi: 10.1083/jcb.104.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Pickett E. B., Saucerman S., McEver R. P., Kunicki T. J., Kieffer N., Newman P. J. Platelet surface glycoproteins. Studies on resting and activated platelets and platelet membrane microparticles in normal subjects, and observations in patients during adult respiratory distress syndrome and cardiac surgery. J Clin Invest. 1986 Aug;78(2):340–348. doi: 10.1172/JCI112582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantgan R. R. A study of the kinetics of ADP-triggered platelet shape change. Blood. 1984 Oct;64(4):896–906. [PubMed] [Google Scholar]
- Hantgan R. R. Fibrin protofibril and fibrinogen binding to ADP-stimulated platelets: evidence for a common mechanism. Biochim Biophys Acta. 1988 Jan 18;968(1):24–35. doi: 10.1016/0167-4889(88)90040-7. [DOI] [PubMed] [Google Scholar]
- Hourdillé P., Hasitz M., Belloc F., Nurden A. T. Immunocytochemical study of the binding of fibrinogen and thrombospondin to ADP- and thrombin-stimulated human platelets. Blood. 1985 Apr;65(4):912–920. [PubMed] [Google Scholar]
- Isenberg W. M., McEver R. P., Phillips D. R., Shuman M. A., Bainton D. F. The platelet fibrinogen receptor: an immunogold-surface replica study of agonist-induced ligand binding and receptor clustering. J Cell Biol. 1987 Jun;104(6):1655–1663. doi: 10.1083/jcb.104.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C., Johnson C., Ramsamooj P., Hantgan R. R. Orientation and specificity of fibrin protofibril binding to ADP-stimulated platelets. Blood. 1988 Dec;72(6):1992–2000. [PubMed] [Google Scholar]
- Lewis J. C., White M. S., Prater T., Taylor R. G., Davis K. S. Ultrastructural analysis of platelets in nonhuman primates. III. Stereo microscopy of microtubules during platelet adhesion and the release reaction. Exp Mol Pathol. 1982 Dec;37(3):370–381. doi: 10.1016/0014-4800(82)90049-1. [DOI] [PubMed] [Google Scholar]
- Loftus J. C., Albrecht R. M. Redistribution of the fibrinogen receptor of human platelets after surface activation. J Cell Biol. 1984 Sep;99(3):822–829. doi: 10.1083/jcb.99.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P. J., Allen R. W., Kahn R. A., Kunicki T. J. Quantitation of membrane glycoprotein IIIa on intact human platelets using the monoclonal antibody, AP-3. Blood. 1985 Jan;65(1):227–232. [PubMed] [Google Scholar]
- Nichols W. L., Gastineau D. A., Solberg L. A., Jr, Mann K. G. Identification of human megakaryocyte coagulation factor V. Blood. 1985 Jun;65(6):1396–1406. [PubMed] [Google Scholar]
- Niiya K., Hodson E., Bader R., Byers-Ward V., Koziol J. A., Plow E. F., Ruggeri Z. M. Increased surface expression of the membrane glycoprotein IIb/IIIa complex induced by platelet activation. Relationship to the binding of fibrinogen and platelet aggregation. Blood. 1987 Aug;70(2):475–483. [PubMed] [Google Scholar]
- Oliver J. A., Albrecht R. M. Colloidal gold labelling of fibrinogen receptors in epinephrine- and ADP-activated platelet suspensions. Scanning Microsc. 1987 Jun;1(2):745–756. [PubMed] [Google Scholar]
- Parker R. I., Gralnick H. R. Identification of platelet glycoprotein IIb/IIIa as the major binding site for released platelet-von Willebrand factor. Blood. 1986 Sep;68(3):732–736. [PubMed] [Google Scholar]
- Peerschke E. I. The platelet fibrinogen receptor. Semin Hematol. 1985 Oct;22(4):241–259. [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Edwards H. H. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980 Jul;86(1):77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Leung L. L., Clark F. Y., Nachman R. L. Thrombin-induced platelet membrane glycoprotein IIb and IIIa complex formation. An electron microscope study. J Exp Med. 1981 Oct 1;154(4):1058–1068. doi: 10.1084/jem.154.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa J. P., Kieffer N., Didry D., Pidard D., Kunicki T. J., Nurden A. T. The human platelet membrane glycoprotein complex GP IIb-IIIa expresses antigenic sites not exposed on the dissociated glycoproteins. Blood. 1984 Dec;64(6):1246–1253. [PubMed] [Google Scholar]
- Santoso S., Zimmermann U., Neppert J., Mueller-Eckhardt C. Receptor patching and capping of platelet membranes induced by monoclonal antibodies. Blood. 1986 Feb;67(2):343–349. [PubMed] [Google Scholar]
- Shattil S. J., Brass L. F., Bennett J. S., Pandhi P. Biochemical and functional consequences of dissociation of the platelet membrane glycoprotein IIb-IIIa complex. Blood. 1985 Jul;66(1):92–98. [PubMed] [Google Scholar]
- Solum N. O. Platelet membrane proteins. Semin Hematol. 1985 Oct;22(4):289–302. [PubMed] [Google Scholar]
- Tetteroo P. A., Lansdorp P. M., Leeksma O. C., von dem Borne A. E. Monoclonal antibodies against human platelet glycoprotein IIIa. Br J Haematol. 1983 Nov;55(3):509–522. doi: 10.1111/j.1365-2141.1983.tb02166.x. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Wencel-Drake J. D., Plow E. F., Zimmerman T. S., Painter R. G., Ginsberg M. H. Immunofluorescent localization of adhesive glycoproteins in resting and thrombin-stimulated platelets. Am J Pathol. 1984 May;115(2):156–164. [PMC free article] [PubMed] [Google Scholar]
- Williams J. E., Hantgan R. R., Hermans J., McDonagh J. Characterization of the inhibition of fibrin assembly by fibrinogen fragment D. Biochem J. 1981 Sep 1;197(3):661–668. doi: 10.1042/bj1970661. [DOI] [PMC free article] [PubMed] [Google Scholar]