Abstract
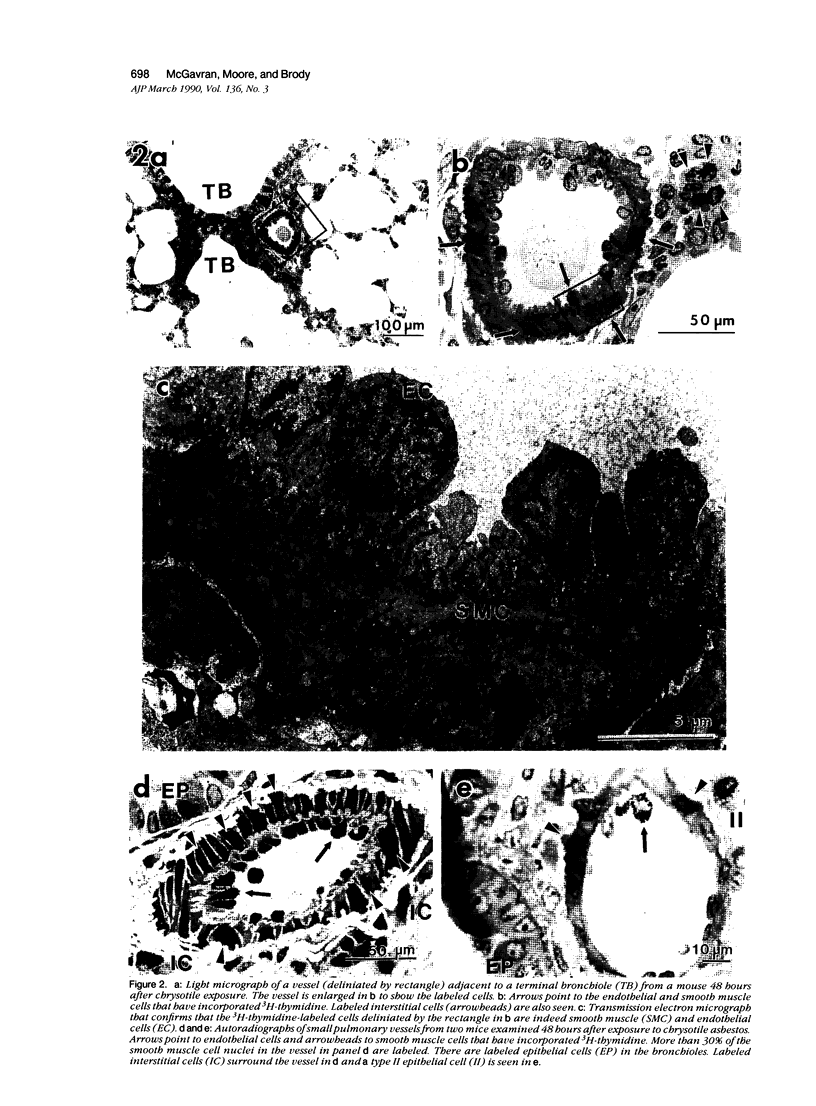
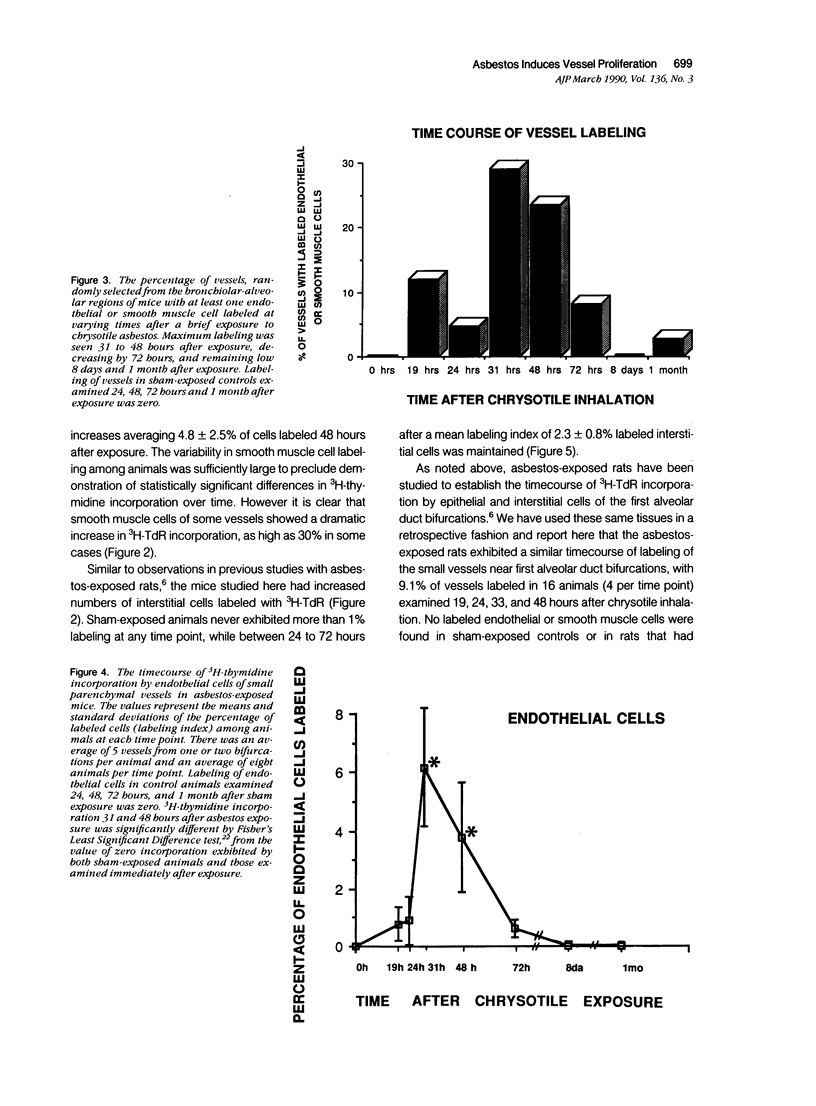
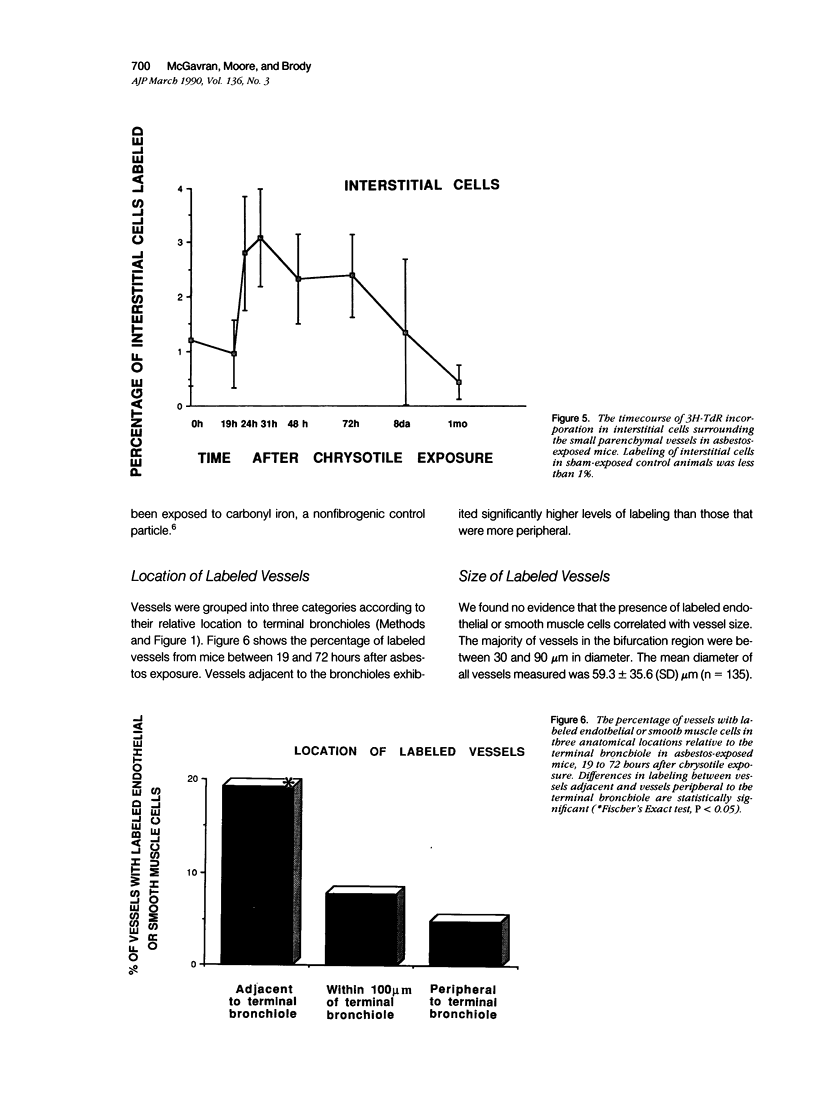
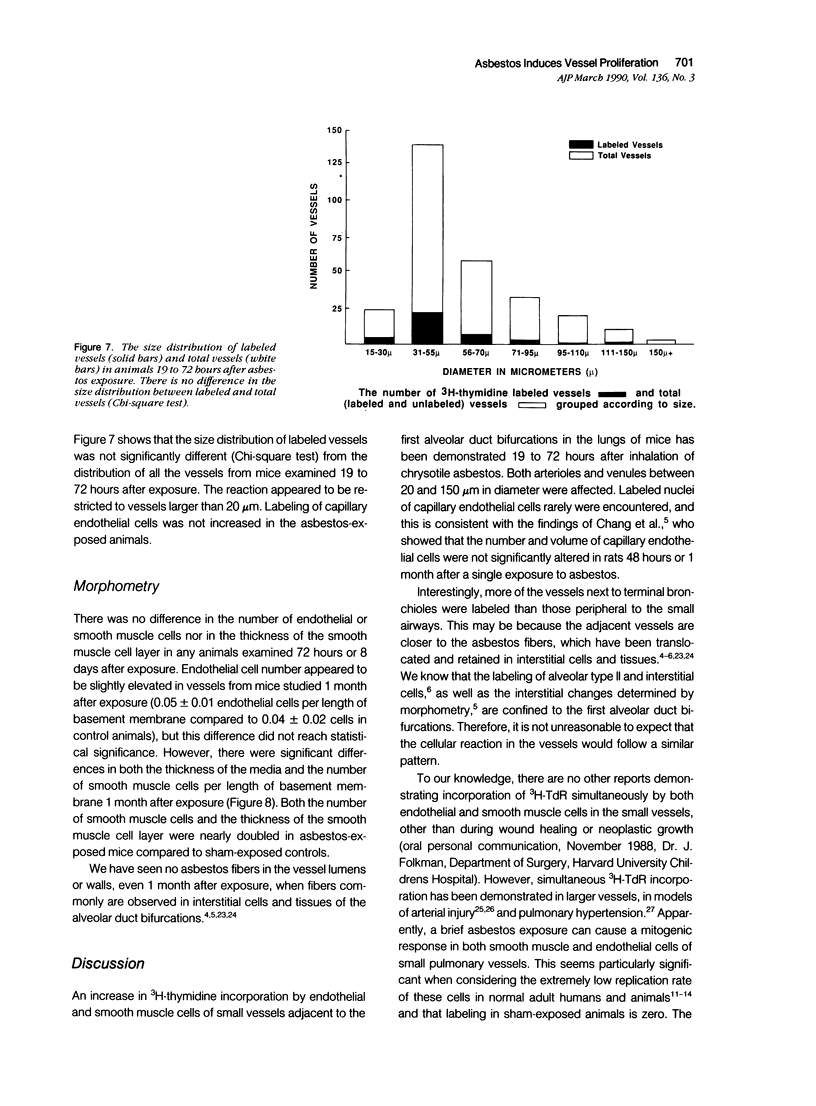
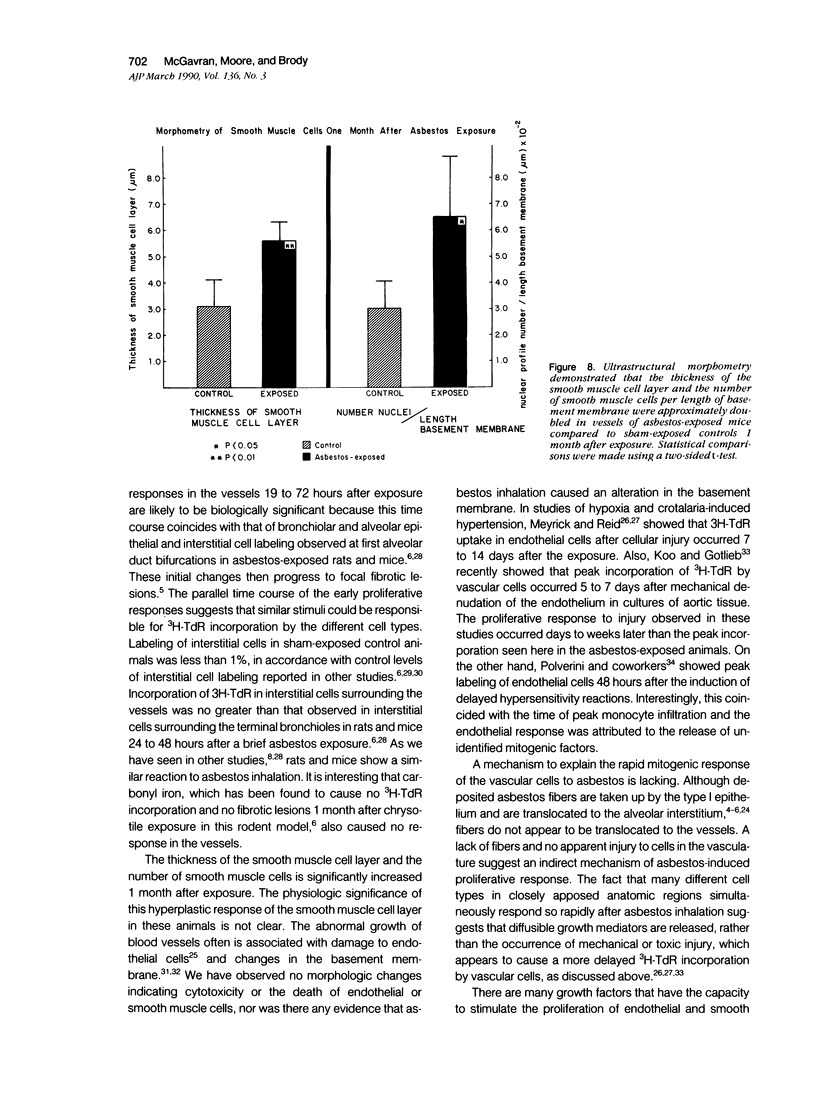
Asbestos inhalation in mice and rats causes a rapid proliferative response in epithelial and interstitial cells, followed by the development of an interstitial lesion at the first alveolar duct bifurcations where fiber deposition and alveolar macrophage accumulation occur. Here we report that endothelial and smooth muscle cells of arterioles and venules near the bifurcations incorporated significantly increased levels of 3H-TdR 19 to 72 hours after chrysotile exposure. As many as 28% of the vessels had labeled cells 31 hours after exposure. No labeled cells were observed in vessels from sham-exposed or iron-exposed controls. This proliferative response resulted in a doubling of both the number of smooth muscle cells and the thickness of the smooth muscle cell layer, determined by ultrastructural morphometry 1 month after exposure. The fact that a variety of cell types incorporates 3H-TdR so rapidly after asbestos inhalation leads us to speculate that the response involves the release of diffusible growth factors.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Crocidolite-induced pulmonary fibrosis in mice. Cytokinetic and biochemical studies. Am J Pathol. 1986 Feb;122(2):261–267. [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachwich P. R., Lynch J. P., 3rd, Larrick J., Spengler M., Kunkel S. L. Tumor necrosis factor production by human sarcoid alveolar macrophages. Am J Pathol. 1986 Dec;125(3):421–425. [PMC free article] [PubMed] [Google Scholar]
- Baird A., Mormède P., Böhlen P. Immunoreactive fibroblast growth factor in cells of peritoneal exudate suggests its identity with macrophage-derived growth factor. Biochem Biophys Res Commun. 1985 Jan 16;126(1):358–364. doi: 10.1016/0006-291x(85)90614-x. [DOI] [PubMed] [Google Scholar]
- Banda M. J., Dwyer K. S., Beckmann A. Wound fluid angiogenesis factor stimulates the directed migration of capillary endothelial cells. J Cell Biochem. 1985;29(3):183–193. doi: 10.1002/jcb.240290303. [DOI] [PubMed] [Google Scholar]
- Barry B. E., Wong K. C., Brody A. R., Crapo J. D. Reaction of rat lungs to inhaled chrysotile asbestos following acute and subchronic exposures. Exp Lung Res. 1983 Jul;5(1):1–21. doi: 10.3109/01902148309061501. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H., Adkins B., Jr, O'Connor R. W. Chrysotile asbestos inhalation in rats: deposition pattern and reaction of alveolar epithelium and pulmonary macrophages. Am Rev Respir Dis. 1981 Jun;123(6):670–679. doi: 10.1164/arrd.1981.123.6.670. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H. Interstitial accumulation of inhaled chrysotile asbestos fibers and consequent formation of microcalcifications. Am J Pathol. 1982 Oct;109(1):107–114. [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Overby L. H. Incorporation of tritiated thymidine by epithelial and interstitial cells in bronchiolar-alveolar regions of asbestos-exposed rats. Am J Pathol. 1989 Jan;134(1):133–140. [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Roe M. W. Deposition pattern of inorganic particles at the alveolar level in the lungs of rats and mice. Am Rev Respir Dis. 1983 Oct;128(4):724–729. doi: 10.1164/arrd.1983.128.4.724. [DOI] [PubMed] [Google Scholar]
- Chang L. Y., Overby L. H., Brody A. R., Crapo J. D. Progressive lung cell reactions and extracellular matrix production after a brief exposure to asbestos. Am J Pathol. 1988 Apr;131(1):156–170. [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Stone R. D., Leung D. Y., Silver I., Hohn D. C., Hunt T. K. Role of macrophages in would healing. Surg Forum. 1976;27(62):16–18. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Coflesky J. T., Adler K. B., Woodcock-Mitchell J., Mitchell J., Evans J. N. Proliferative changes in the pulmonary arterial wall during short-term hyperoxic injury to the lung. Am J Pathol. 1988 Sep;132(3):563–573. [PMC free article] [PubMed] [Google Scholar]
- D'Amore P. A., Glaser B. M., Brunson S. K., Fenselau A. H. Angiogenic activity from bovine retina: partial purification and characterization. Proc Natl Acad Sci U S A. 1981 May;78(5):3068–3072. doi: 10.1073/pnas.78.5.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann R. F., Cohen I. K., Kaplan A. M. Effect of macrophages on fibroblast DNA synthesis and proliferation. Proc Soc Exp Biol Med. 1982 Apr;169(4):445–451. doi: 10.3181/00379727-169-41373. [DOI] [PubMed] [Google Scholar]
- Dilley R. J., McGeachie J. K., Prendergast F. J. A review of the proliferative behaviour, morphology and phenotypes of vascular smooth muscle. Atherosclerosis. 1987 Feb;63(2-3):99–107. doi: 10.1016/0021-9150(87)90109-2. [DOI] [PubMed] [Google Scholar]
- Engerman R. L., Pfaffenbach D., Davis M. D. Cell turnover of capillaries. Lab Invest. 1967 Dec;17(6):738–743. [PubMed] [Google Scholar]
- Evans M. J., Bils R. F. Identification of cells labeled with tritiated thymidine in the pulmonary alveolar walls of the mouse. Am Rev Respir Dis. 1969 Sep;100(3):372–378. doi: 10.1164/arrd.1969.100.3.372. [DOI] [PubMed] [Google Scholar]
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Vascular physiology. A family of angiogenic peptides. Nature. 1987 Oct 22;329(6141):671–672. doi: 10.1038/329671a0. [DOI] [PubMed] [Google Scholar]
- Form D. M., Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983 Feb;172(2):214–218. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- Form D. M., Pratt B. M., Madri J. A. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986 Nov;55(5):521–530. [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ill C. Extracellular matrix and control of proliferation of vascular endothelial cells. J Clin Invest. 1980 Jun;65(6):1351–1364. doi: 10.1172/JCI109799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Hirst D. G., Denekamp J., Hobson B. Proliferation studies of the endothelial and smooth muscle cells of the mouse mesentery after irradiation. Cell Tissue Kinet. 1980 Jan;13(1):91–104. doi: 10.1111/j.1365-2184.1980.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Scheuenstuhl H., Halliday B. J., Werb Z., Banda M. J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983 Sep 23;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Koo E. W., Gotlieb A. I. Endothelial stimulation of intimal cell proliferation in a porcine aortic organ culture. Am J Pathol. 1989 Mar;134(3):497–503. [PMC free article] [PubMed] [Google Scholar]
- Kumar R. K., Bennett R. A., Brody A. R. A homologue of platelet-derived growth factor produced by rat alveolar macrophages. FASEB J. 1988 Apr;2(7):2272–2277. doi: 10.1096/fasebj.2.7.3280379. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Polverini P. J., Shepard H. M., Wiseman D. M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987 Oct 15;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. A macrophage-dependent factor that stimulates the proliferation of fibroblasts in vitro. Am J Pathol. 1976 Sep;84(3):501–514. [PMC free article] [PubMed] [Google Scholar]
- Leslie C. C., McCormick-Shannon K., Cook J. L., Mason R. J. Macrophages stimulate DNA synthesis in rat alveolar type II cells. Am Rev Respir Dis. 1985 Dec;132(6):1246–1252. doi: 10.1164/arrd.1985.132.6.1246. [DOI] [PubMed] [Google Scholar]
- Libby P., Wyler D. J., Janicka M. W., Dinarello C. A. Differential effects of human interleukin-1 on growth of human fibroblasts and vascular smooth muscle cells. Arteriosclerosis. 1985 Mar-Apr;5(2):186–191. doi: 10.1161/01.atv.5.2.186. [DOI] [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Meyrick B. O., Reid L. M. Crotalaria-induced pulmonary hypertension. Uptake of 3H-thymidine by the cells of the pulmonary circulation and alveolar walls. Am J Pathol. 1982 Jan;106(1):84–94. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Hypoxia and incorporation of 3H-thymidine by cells of the rat pulmonary arteries and alveolar wall. Am J Pathol. 1979 Jul;96(1):51–70. [PMC free article] [PubMed] [Google Scholar]
- Mornex J. F., Martinet Y., Yamauchi K., Bitterman P. B., Grotendorst G. R., Chytil-Weir A., Martin G. R., Crystal R. G. Spontaneous expression of the c-sis gene and release of a platelet-derived growth factorlike molecule by human alveolar macrophages. J Clin Invest. 1986 Jul;78(1):61–66. doi: 10.1172/JCI112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe T., Takaku F. A macrophage factor that stimulates the proliferation of vascular endothelial cells. Biochem Biophys Res Commun. 1986 Jan 14;134(1):344–350. doi: 10.1016/0006-291x(86)90569-3. [DOI] [PubMed] [Google Scholar]
- Palmberg L., Claesson H. E., Thyberg J. Leukotrienes stimulate initiation of DNA synthesis in cultured arterial smooth muscle cells. J Cell Sci. 1987 Sep;88(Pt 2):151–159. doi: 10.1242/jcs.88.2.151. [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Pratt P. C., Brody A. R., Crapo J. D. Fiber localization and its relationship to lung reaction in rats after chronic inhalation of chrysotile asbestos. Am J Pathol. 1984 Dec;117(3):484–498. [PMC free article] [PubMed] [Google Scholar]
- Polverini P. J., Cotran P. S., Gimbrone M. A., Jr, Unanue E. R. Activated macrophages induce vascular proliferation. Nature. 1977 Oct 27;269(5631):804–806. doi: 10.1038/269804a0. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Cotran R. S., Sholley M. M. Endothelial proliferation in the delayed hypersensitivity reaction: an autoradiographic study. J Immunol. 1977 Feb;118(2):529–532. [PubMed] [Google Scholar]
- Polverini P. J., Leibovich S. J. Induction of neovascularization and nonlymphoid mesenchymal cell proliferation by macrophage cell lines. J Leukoc Biol. 1985 Mar;37(3):279–288. doi: 10.1002/jlb.37.3.279. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Leibovich S. J. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest. 1984 Dec;51(6):635–642. [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Oliver C. N., Lepe-Zuniga J. L., Green I., Gery I. Silica-stimulated monocytes release fibroblast proliferation factors identical to interleukin 1. A potential role for interleukin 1 in the pathogenesis of silicosis. J Clin Invest. 1984 May;73(5):1462–1472. doi: 10.1172/JCI111350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Thakral K. K., Goodson W. H., 3rd, Hunt T. K. Stimulation of wound blood vessel growth by wound macrophages. J Surg Res. 1979 Apr;26(4):430–436. doi: 10.1016/0022-4804(79)90031-3. [DOI] [PubMed] [Google Scholar]
- Wagner J. C., Berry G., Skidmore J. W., Timbrell V. The effects of the inhalation of asbestos in rats. Br J Cancer. 1974 Mar;29(3):252–269. doi: 10.1038/bjc.1974.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit D. B., Chang L. Y., Hill L. H., Hook G. E., Crapo J. D., Brody A. R. Pulmonary macrophage accumulation and asbestos-induced lesions at sites of fiber deposition. Am Rev Respir Dis. 1984 Feb;129(2):301–310. [PubMed] [Google Scholar]
- Warheit D. B., George G., Hill L. H., Snyderman R., Brody A. R. Inhaled asbestos activates a complement-dependent chemoattractant for macrophages. Lab Invest. 1985 May;52(5):505–514. [PubMed] [Google Scholar]
- Warheit D. B., Hill L. H., George G., Brody A. R. Time course of chemotactic factor generation and the corresponding macrophage response to asbestos inhalation. Am Rev Respir Dis. 1986 Jul;134(1):128–133. doi: 10.1164/arrd.1986.134.1.128. [DOI] [PubMed] [Google Scholar]