Abstract
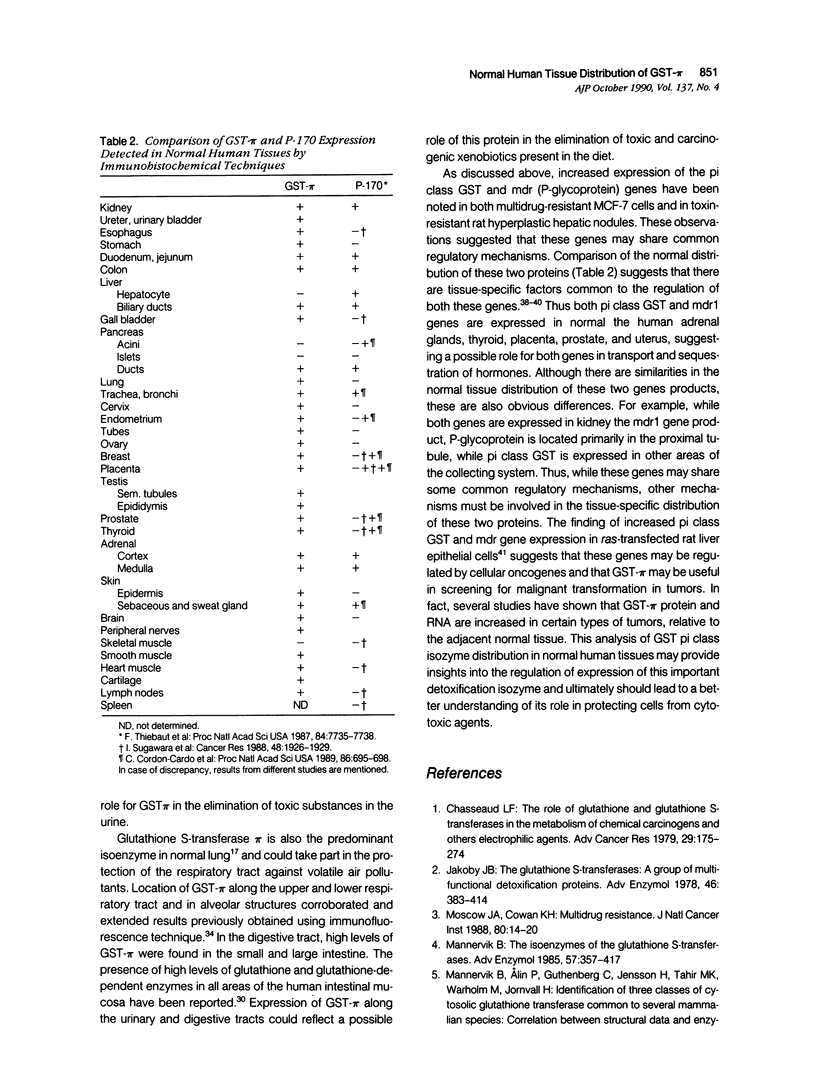
Glutathione S-transferases (GSTs), a family of isoenzymes that play an important role in protecting cells from cytotoxic and carcinogenic agents, can be separated by biochemical and immunologic characteristics into three distinct classes named alpha, mu, and pi. Previous studies have indicated that there is marked heterogeneity in the expression of different GST isoenzymes in different normal and malignant tissues. To better understand the regulation of the human pi class glutathione S-transferase isoenzyme (GST-pi), the tissue distribution of this protein wa studied by an immunohistochemical technique using an anti-GST-pi polyclonal antibody in normal paraffin-embedded human tissues. These studies indicate that there is a broad distribution of GST-pi in normal human tissues and establish a precise localization within the different organs studied. GST-pi was expressed predominantly in normal epithelial cells of the urinary, digestive, and respiratory tracts, suggesting a possible role for GST-pi in detoxication and elimination of toxic substances. Previous studies have indicated that GST-pi and the putative drug efflux pump P-glycoprotein are both overexpressed in multidrug-resistant human breast cancer cells and in xenobiotic resistant preneoplastic rat hyperplastic liver nodules. Results from this study indicate that there are also similarities between the normal tissue distribution GST-pi and that previously reported for mammalian P-glycoprotein, particularly in secretory epithelia. This finding suggests that these two gene products, which have been implicated in the development of resistance to cytotoxic drugs, may be coregulated in normal and malignant cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Burt R. K., Garfield S., Johnson K., Thorgeirsson S. S. Transformation of rat liver epithelial cells with v-H-ras or v-raf causes expression of MDR-1, glutathione-S-transferase-P and increased resistance to cytotoxic chemicals. Carcinogenesis. 1988 Dec;9(12):2329–2332. doi: 10.1093/carcin/9.12.2329. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Casals D., Rittman-Grauer L., Biedler J. L., Melamed M. R., Bertino J. R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989 Jan;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ilio C., Del Boccio G., Aceto A., Casaccia R., Mucilli F., Federici G. Elevation of glutathione transferase activity in human lung tumor. Carcinogenesis. 1988 Feb;9(2):335–340. doi: 10.1093/carcin/9.2.335. [DOI] [PubMed] [Google Scholar]
- Di Ilio C., Del Boccio G., Aceto A., Federici G. Alteration of glutathione transferase isoenzyme concentrations in human renal carcinoma. Carcinogenesis. 1987 Jun;8(6):861–864. doi: 10.1093/carcin/8.6.861. [DOI] [PubMed] [Google Scholar]
- Eimoto H., Tsutsumi M., Nakajima A., Yamamoto K., Takashima Y., Maruyama H., Konishi Y. Expression of the glutathione S-transferase placental form in human lung carcinomas. Carcinogenesis. 1988 Dec;9(12):2325–2327. doi: 10.1093/carcin/9.12.2325. [DOI] [PubMed] [Google Scholar]
- Fairchild C. R., Ivy S. P., Kao-Shan C. S., Whang-Peng J., Rosen N., Israel M. A., Melera P. W., Cowan K. H., Goldsmith M. E. Isolation of amplified and overexpressed DNA sequences from adriamycin-resistant human breast cancer cells. Cancer Res. 1987 Oct 1;47(19):5141–5148. [PubMed] [Google Scholar]
- Farber E., Parker S., Gruenstein M. The resistance of putative premalignant liver cell populations, hyperplastic nodules, to the acute cytotoxic effects of some hepatocarcinogens. Cancer Res. 1976 Nov;36(11 Pt 1):3879–3887. [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Jensson H., Eriksson L. C., Mannervik B. Selective expression of glutathione transferase isoenzymes in chemically induced preneoplastic rat hepatocyte nodules. FEBS Lett. 1985 Jul 22;187(1):115–120. doi: 10.1016/0014-5793(85)81225-4. [DOI] [PubMed] [Google Scholar]
- Kano T., Sakai M., Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987 Nov 1;47(21):5626–5630. [PubMed] [Google Scholar]
- Kodate C., Fukushi A., Narita T., Kudo H., Soma Y., Sato K. Human placental form of glutathione S-transferase (GST-pi) as a new immunohistochemical marker for human colonic carcinoma. Jpn J Cancer Res. 1986 Mar;77(3):226–229. [PubMed] [Google Scholar]
- Lutzky J., Astor M. B., Taub R. N., Baker M. A., Bhalla K., Gervasoni J. E., Jr, Rosado M., Stewart V., Krishna S., Hindenburg A. A. Role of glutathione and dependent enzymes in anthracycline-resistant HL60/AR cells. Cancer Res. 1989 Aug 1;49(15):4120–4125. [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Beale D., Tan K. H., Coles B., Ketterer B. Glutathione transferases in primary rat hepatomas: the isolation of a form with GSH peroxidase activity. FEBS Lett. 1985 May 6;184(1):139–143. doi: 10.1016/0014-5793(85)80670-0. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Satoh K., Kitahara A., Sato K., Ito N. A protein cross-reacting immunohistochemically with rat glutathione S-transferase placental form as a marker for preneoplasia in Syrian hamster pancreatic and hepatocarcinogenesis. Jpn J Cancer Res. 1985 Jan;76(1):1–4. [PubMed] [Google Scholar]
- Moscow J. A., Cowan K. H. Multidrug resistance. J Natl Cancer Inst. 1988 Mar 2;80(1):14–20. doi: 10.1093/jnci/80.1.14. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Fairchild C. R., Madden M. J., Ransom D. T., Wieand H. S., O'Brien E. E., Poplack D. G., Cossman J., Myers C. E., Cowan K. H. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989 Mar 15;49(6):1422–1428. [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Goldsmith M. E., Whang-Peng J., Vickers P. J., Poisson R., Legault-Poisson S., Myers C. E., Cowan K. H. Isolation of the human anionic glutathione S-transferase cDNA and the relation of its gene expression to estrogen-receptor content in primary breast cancer. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6518–6522. doi: 10.1073/pnas.85.17.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitsu Y., Takahashi Y., Saito T., Hirata Y., Arisato N., Maruyama H., Kohgo Y., Listowsky I. Serum glutathione-S-transferase-pi as a tumor marker for gastrointestinal malignancies. Cancer. 1989 Jan 15;63(2):317–323. doi: 10.1002/1097-0142(19890115)63:2<317::aid-cncr2820630219>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Sato K., Kitahara A., Satoh K., Ishikawa T., Tatematsu M., Ito N. The placental form of glutathione S-transferase as a new marker protein for preneoplasia in rat chemical hepatocarcinogenesis. Gan. 1984 Mar;75(3):199–202. [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Soma Y., Inaba Y., Hatayama I., Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea T. C., Kelley S. L., Henner W. D. Identification of an anionic form of glutathione transferase present in many human tumors and human tumor cell lines. Cancer Res. 1988 Feb 1;48(3):527–533. [PubMed] [Google Scholar]
- Shiratori Y., Soma Y., Maruyama H., Sato S., Takano A., Sato K. Immunohistochemical detection of the placental form of glutathione S-transferase in dysplastic and neoplastic human uterine cervix lesions. Cancer Res. 1987 Dec 15;47(24 Pt 1):6806–6809. [PubMed] [Google Scholar]
- Siegers C. P., Böse-Younes H., Thies E., Hoppenkamps R., Younes M. Glutathione and GSH-dependent enzymes in the tumorous and nontumorous mucosa of the human colon and rectum. J Cancer Res Clin Oncol. 1984;107(3):238–241. doi: 10.1007/BF01032615. [DOI] [PubMed] [Google Scholar]
- Singh S. V., Leal T., Ansari G. A., Awasthi Y. C. Purification and characterization of glutathione S-transferases of human kidney. Biochem J. 1987 Aug 15;246(1):179–186. doi: 10.1042/bj2460179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma Y., Satoh K., Sato K. Purification and subunit-structural and immunological characterization of five glutathione S-transferases in human liver, and the acidic form as a hepatic tumor marker. Biochim Biophys Acta. 1986 Feb 14;869(3):247–258. doi: 10.1016/0167-4838(86)90064-6. [DOI] [PubMed] [Google Scholar]
- Strange R. C., Davis B. A., Faulder C. G., Cotton W., Bain A. D., Hopkinson D. A., Hume R. The human glutathione S-transferases: developmental aspects of the GST1, GST2, and GST3 loci. Biochem Genet. 1985 Dec;23(11-12):1011–1028. doi: 10.1007/BF00499944. [DOI] [PubMed] [Google Scholar]
- Sugawara I., Kataoka I., Morishita Y., Hamada H., Tsuruo T., Itoyama S., Mori S. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res. 1988 Apr 1;48(7):1926–1929. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Townsend A. J., Goldsmith M. E., Pickett C. B., Cowan K. H. Isolation, characterization, and expression in Escherichia coli of two murine Mu class glutathione S-transferase cDNAs homologous to the rat subunits 3 (Yb1) and 4 (Yb2). J Biol Chem. 1989 Dec 25;264(36):21582–21590. [PubMed] [Google Scholar]
- Whelan R. D., Hosking L. K., Townsend A. J., Cowan K. H., Hill B. T. Differential increases in glutathione S-transferase activities in a range of multidrug-resistant human tumor cell lines. Cancer Commun. 1989;1(6):359–365. doi: 10.3727/095535489820875057. [DOI] [PubMed] [Google Scholar]
- Whyte W. L., Pong R. S., Buckpitt A. R. Glutathione and glutathione S-transferases in the urinary bladder of different species. Biochem Pharmacol. 1984 Jun 1;33(11):1813–1816. doi: 10.1016/0006-2952(84)90356-3. [DOI] [PubMed] [Google Scholar]
- di Ilio C., Sacchetta P., del Boccio G., la Rovere G., Federici G. Glutathione peroxidase, glutathione S-transferase and glutathione reductase activities in normal and neoplastic human breast tissue. Cancer Lett. 1985 Oct;29(1):37–42. doi: 10.1016/0304-3835(85)90120-x. [DOI] [PubMed] [Google Scholar]