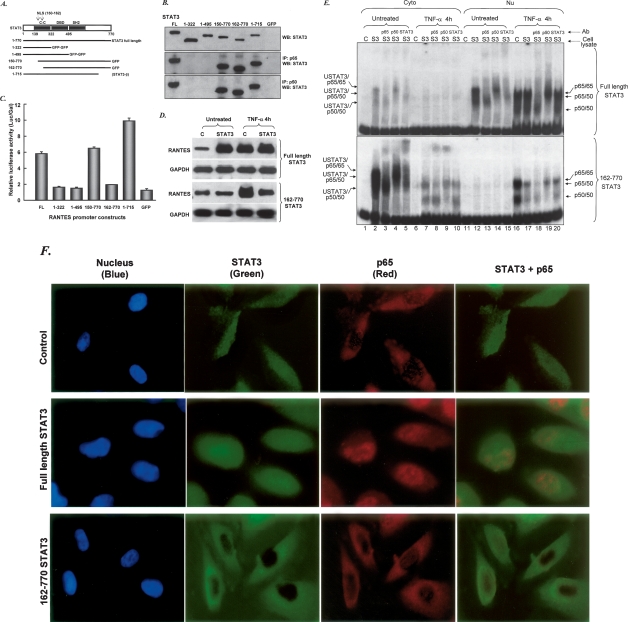
Figure 3.
The SH2 and NLS domains of STAT3 are required for interaction with p65 and p50 and for up-regulation of the RANTES promoter. (A) STAT3 domains and deletion constructs. (B) PC3 cells were transfected with expression constructs for N- and C-terminal deletions of STAT3 and, 48 h later, whole-cell lysates were prepared and assayed by coimmunoprecipitation with anti-p65 or anti-p50 and by the Western method with anti-STAT3. Three different antibodies that react with the N-terminal, C-terminal, and middle portions of STAT3 were used. (C) Expression constructs for N- and C-terminal deletions of STAT3 were cotransfected into PC3 cells with pGL2-220 and pCH110, and the cells were harvested for luciferase assays 48 h later. (D) Northern analysis. Total RNAs (20 μg per lane) from hTERT-HME1 cells untreated or treated with IL-6 were analyzed by the Northern method. (E) DNA-binding assays. hTERT-HME1 cells expressing a high level of full-length STAT3 or 162–770 truncated STAT3 were untreated or treated with TNF-α for 4 h. EMSAs were performed with cytoplasmic and nuclear fractions. A DNA fragment of the human RANTES promoter, bases −58 to −29, containing a κB element, was used as the labeled probe. Assays were performed by adding equal amounts of proteins. (C) hTERT-HME1 control cells; (S3) hTERT-HME1 cells expressing a high level of STAT3. (F) hTERT-HME1 cells expressing a high level of full-length or 162–770 truncated STAT3 were grown on cover slips and stained with primary antibodies directed against STAT3 and p65. Following treatment with DAPI (blue nuclear stain) and fluorescent secondary antibodies for STAT3 (green) and p65 (red), the cells were examined by using confocal microscopy. The yellow pixels in the composite image demonstrate the close association of the two proteins.