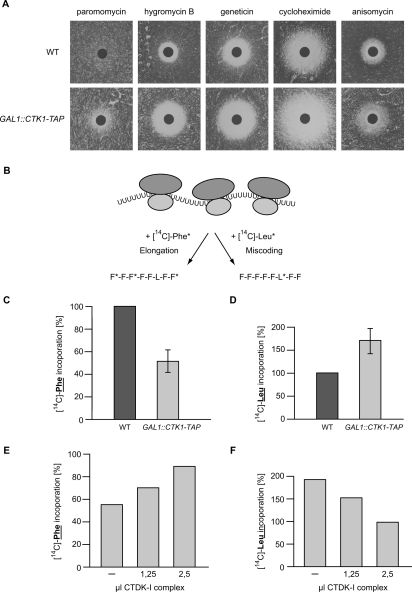
Figure 3.
Ctk1 plays a role in decoding fidelity. (A) Ctk1-depleted cells are sensitive to translation inhibitors. GAL1∷CTK1-TAP and wild-type (WT) cells were grown for 18 h in YPD and then plated on YPD plates, on which a filter containing paromomycin, hygromycin B, geneticin, cycloheximide, or anisomycin was placed. The size of the halo indicates the sensitivity of the strain toward the drug. (B) Schematic showing the poly(U) assay. The rate of translation elongation was determined by measuring the incorporation of radioactively labeled phenylalanine (F*; left arrow), and the rate of miscoding was measured by the incorporation of radioactively labeled leucine (L*; right arrow) when poly(U) RNA was used as a template in the translation assay. (C) Depletion of Ctk1 leads to a decrease in the efficiency of translation elongation. The incorporation of [14C]-labeled phenylalanine into the polypeptide chain of nuclease-treated extracts prepared from wild-type (WT) and GAL1∷CTK1-TAP cells after 18 h of depletion in glucose-containing medium was measured. The activity of wild-type cells was set to 100%. (D) Loss of Ctk1 function leads to an increase in the frequency of miscoding. Cells depleted for Ctk1 by growth in glucose-containing medium (YPD) for 18 h show an increase in the frequency of miscoding as measured by the incorporation of [14C]-labeled leucine. (E,F) The defect of Ctk1-depleted cells in translation elongation (E) and translational accuracy (F) can be rescued by reconstitution with purified CTDK-I complex in a dose-dependent manner. Purified CTDK-I complex was added to extracts depleted for Ctk1 for 18 h, and the efficiency of translation elongation and the miscoding frequency was measured as in C and D, respectively. As negative control, an eluate from a mock purification of a nontagged wild-type strain was added to the translation assays, and the activities of the CTDK-I-treated extracts were calculated relative to the mock-treated ones. One representative experiment of three independent experiments is shown.