TABLE 1.
Reactions and relative rate constants of ODEs modeling of the regulatory network of mitochondrial apoptosis
| Description of reactions | ||
|---|---|---|
| Act + InBax | 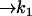 |
Act + AcBax |
| AcBax | 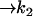 |
InBax |
| AcBax + Bcl2 | 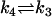 |
AcBaxBcl2 |
| Act + Bcl2 | 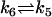 |
ActBcl2 |
| AcBax + ActBcl2 | 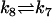 |
Act + AcBaxBcl2 |
| 4 AcBax | 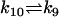 |
Bax4 |
| with | ||
| Rate constants | Initial conditions |
|---|---|
| k1 =0.5 μM−1 s−1 | Actmito = 0 × 10−4 mM |
| k2 = 0.1 s−1 | Baxmito = 2 × 10−4 mM |
| k3 = 2 μM−1 s−1 | Bcl2mito = 1 × 10−4 mM |
| k4 = 0.001 s−1 | Actcytosol = 1 × 10−4 mM |
| k5 = 3 μM−1 s−1 | Baxcytosol = 2 × 10−4 mM |
| k6 = 0.04 s−1 | Bcl2cytosol = 0 × 10−4 mM |
| k7 = 2 μM−1 s−1 | |
| k8 = 0 μM−1 s−1 | |
| k9 = 2 μM−1 s−1 | |
| k10 = 0 s−1 |
For clarity, abbreviations in Table 2 are used for description of reactions. We set kbcl2 (the rate of non-free Bcl2 shifting to free Bcl2; for details, see Model and Methods) equal to k6 assuming that free Bcl2 originate from AcBaxBcl2 at the same rate with ActBcl2. The parameter k1, k3, k4, k5, k6, k8, and k9 are set up to with one order-of-magnitude difference with respect to those used by Hua et al. (43) in their modeling of the effects of Bcl2-level on Fas signaling-induced caspase-3 activation. The ratio of k3 to k4 ensures an equilibrium constant of 1e-8M from (32), as well as those used for defining k5 and k6. The value of k2, k7, and k8 are suitably chosen to reflect the experimentally known features (13,32,33). To reflect the irreversibility of the process of Bax-polymerization (35), k10 is step to 0 in our simulations. The concentrations of relative Bcl-2 family proteins in initial conditions are set up according to experimental data given by Kuwana et al. (42) and with one order-of-magnitude difference with respect to those used by Hua et al. (43).
